BMS-708163 (Avagacestat)γ-secretase inhibitor CAS# 1146699-66-2 |
- DAPT (GSI-IX)
Catalog No.:BCC3618
CAS No.:208255-80-5
- MK-0752
Catalog No.:BCC2090
CAS No.:471905-41-6
- Flurizan
Catalog No.:BCC2342
CAS No.:51543-40-9
- JLK 6
Catalog No.:BCC2343
CAS No.:62252-26-0
- Begacestat
Catalog No.:BCC2346
CAS No.:769169-27-9
- E 2012
Catalog No.:BCC1540
CAS No.:870843-42-8
Quality Control & MSDS
Number of papers citing our products

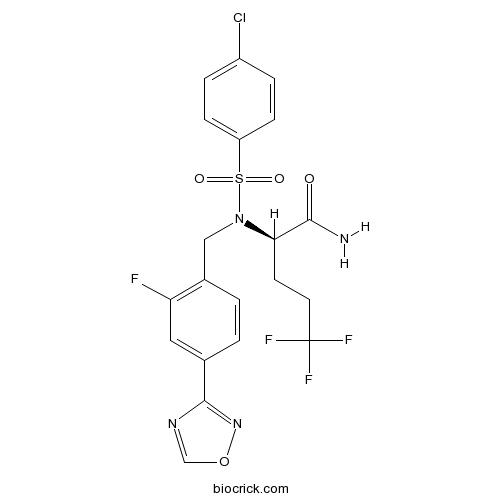
Chemical structure

3D structure
| Cas No. | 1146699-66-2 | SDF | Download SDF |
| PubChem ID | 46883536 | Appearance | Powder |
| Formula | C20H17ClF4N4O4S | M.Wt | 520.88 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | Avagacestat | ||
| Solubility | DMSO : ≥ 100 mg/mL (191.98 mM) H2O : < 0.1 mg/mL (insoluble) *"≥" means soluble, but saturation unknown. | ||
| Chemical Name | (2R)-2-[(4-chlorophenyl)sulfonyl-[[2-fluoro-4-(1,2,4-oxadiazol-3-yl)phenyl]methyl]amino]-5,5,5-trifluoropentanamide | ||
| SMILES | C1=CC(=CC=C1S(=O)(=O)N(CC2=C(C=C(C=C2)C3=NOC=N3)F)C(CCC(F)(F)F)C(=O)N)Cl | ||
| Standard InChIKey | XEAOPVUAMONVLA-QGZVFWFLSA-N | ||
| Standard InChI | InChI=1S/C20H17ClF4N4O4S/c21-14-3-5-15(6-4-14)34(31,32)29(17(18(26)30)7-8-20(23,24)25)10-13-2-1-12(9-16(13)22)19-27-11-33-28-19/h1-6,9,11,17H,7-8,10H2,(H2,26,30)/t17-/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | BMS-708163 is a potent, selective, orally bioavailable inhibitor of γ-secretase with IC50 value of 0.3 nM and 0.27 nM for Aβ40 and Aβ42, respectively. | |||||
| Targets | γ-secretase | γ-secretase | ||||
| IC50 | 0.3 nM (Aβ40) | 0.27 nM (Aβ42) | ||||

BMS-708163 (Avagacestat) Dilution Calculator

BMS-708163 (Avagacestat) Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.9198 mL | 9.5991 mL | 19.1983 mL | 38.3966 mL | 47.9957 mL |
| 5 mM | 0.384 mL | 1.9198 mL | 3.8397 mL | 7.6793 mL | 9.5991 mL |
| 10 mM | 0.192 mL | 0.9599 mL | 1.9198 mL | 3.8397 mL | 4.7996 mL |
| 50 mM | 0.0384 mL | 0.192 mL | 0.384 mL | 0.7679 mL | 0.9599 mL |
| 100 mM | 0.0192 mL | 0.096 mL | 0.192 mL | 0.384 mL | 0.48 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
BMS-708163 is a potent selective and orally bioavailable inhibitor of γ-secretase with IC50 value of 0.3nM [1].
The cleavage of amyloid precusor protein APP by γ-secretase causes the production of Aβ40 and Aβ42, which are cytotoxic and cause AD. In vitro assay shows BMS-708163 inhibits the formation of Aβ40 and Aβ42 in H4-8Sw cells. It also inhibits the human recombinant CYPs in vitro with IC50 value of 20μM. Notch receptor is another substrate of γ-secretase. The inhibition of Notch signal pathway results in some side effects. It is shown that the activity of BMS-708163 to inhibit Notch is 190-fold weaker than to APP. In female rats, oral administration of BMS-708163 significantly reduces the production of Aβ in plasma and brain at 10mg/kg and 100mg/kg. In addition, BMS-708163 also reduces Aβ in brain and CSF in male beagle dogs [1].
References:
[1] Gillman KW, Starrett JE Jr, Parker MF, Xie K, Bronson JJ, Marcin LR, McElhone KE, Bergstrom CP, Mate RA, Williams R, Meredith JE Jr, Burton CR, Barten DM, Toyn JH, Roberts SB, Lentz KA, Houston JG, Zaczek R, Albright CF, Decicco CP, Macor JE, Olson RE. Discovery and Evaluation of BMS-708163, a Potent, Selective and Orally Bioavailable γ-Secretase Inhibitor. ACS Med Chem Lett. 2010 Mar 22;1(3):120-4.
- SNS-314 Mesylate
Catalog No.:BCC2177
CAS No.:1146618-41-8
- 3,4-Di-O-caffeoylquinic acid methyl ester
Catalog No.:BCN6490
CAS No.:114637-83-1
- 17alpha-Thevebioside
Catalog No.:BCN6026
CAS No.:114613-59-1
- Moracin T
Catalog No.:BCN3291
CAS No.:1146113-27-0
- Kisspeptin 234
Catalog No.:BCC6077
CAS No.:1145998-81-7
- Soyasaponin Ba
Catalog No.:BCN2854
CAS No.:114590-20-4
- Thevebioside
Catalog No.:BCN6025
CAS No.:114586-47-9
- Coronadiene
Catalog No.:BCN3683
CAS No.:1145689-64-0
- Ganoderiol F
Catalog No.:BCN6024
CAS No.:114567-47-4
- Boeravinone B
Catalog No.:BCN6466
CAS No.:114567-34-9
- Galanin (1-29) (rat, mouse)
Catalog No.:BCC5928
CAS No.:114547-31-8
- Regelidine
Catalog No.:BCN3094
CAS No.:114542-54-0
- J 147
Catalog No.:BCC6360
CAS No.:1146963-51-0
- Columbianetin
Catalog No.:BCN8502
CAS No.:1147-29-1
- 2-(2-Aminobenzoyl)-benzoic acid
Catalog No.:BCC8476
CAS No.:1147-43-9
- Methyl 4'-methylbiphenyl-2-carboxylate
Catalog No.:BCC9040
CAS No.:114772-34-8
- Methyl 4'-bromomethyl biphenyl-2-carboxylate
Catalog No.:BCC9039
CAS No.:114772-38-2
- tert-Butyl4'-(bromomethyl)biphenyl-2-carboxylate
Catalog No.:BCC9164
CAS No.:114772-40-6
- 4-Bromomethyl-2-cyanobiphenyl
Catalog No.:BCC8702
CAS No.:114772-54-2
- Losartan
Catalog No.:BCC4090
CAS No.:114798-26-4
- Z-Pro-OH
Catalog No.:BCC2754
CAS No.:1148-11-4
- ST-836
Catalog No.:BCC1968
CAS No.:1148156-63-1
- Boc-Phe(2-F)-OH
Catalog No.:BCC3223
CAS No.:114873-00-6
- Boc-Phe(3-Cl)-OH
Catalog No.:BCC2640
CAS No.:114873-03-9
A placebo-controlled, multiple ascending dose study to evaluate the safety, pharmacokinetics and pharmacodynamics of avagacestat (BMS-708163) in healthy young and elderly subjects.[Pubmed:23018531]
Clin Pharmacokinet. 2012 Oct 1;51(10):681-93.
BACKGROUND AND OBJECTIVES: Avagacestat is an orally active gamma-secretase inhibitor that selectively inhibits amyloid beta (Abeta) synthesis in cell culture and animal models. The objective of the current study was to assess the pharmacokinetics, pharmacodynamics, safety and tolerability of multiple doses of avagacestat over 28 days in healthy young men and elderly men and women in a placebo-controlled, sequential-panel, ascending multiple-dose study. METHODS: Thirty-three young men were assigned to four serial dose groups of avagacestat 15, 50, 100 or 150 mg (n = 6-7 per dose), or placebo (n = 2 per dose panel; 8 subjects total) once daily for 28 days. Elderly men and women were assigned to serial dose groups of avagacestat 50 mg and then 100 mg (n = 7 men, 6 women) or placebo (n = 2 men, 2 women) once daily for 14 days per dose level. RESULTS: Avagacestat was rapidly absorbed, had a terminal elimination half-life of 38-65 h, and reached a steady-state concentration by day 10 of daily dosing. Exposure in young subjects increased in proportion to dose. There were no apparent differences in steady-state area under the plasma concentration-time curve between young and elderly subjects; however, elderly subjects demonstrated a higher maximum plasma concentration for avagacestat. Doses of avagacestat >50 mg/day reduced steady-state trough concentrations of CSF Abeta(1-38), Abeta(1-40) and Abeta(1-42) in a dose-dependent fashion over 28 days of daily dosing. There were no signs of potential Notch-related dose-limiting toxicities. CONCLUSION: The results support continued evaluation of avagacestat in an elderly target population with predementia and mild to moderate Alzheimer's disease.
Effects of single doses of avagacestat (BMS-708163) on cerebrospinal fluid Abeta levels in healthy young men.[Pubmed:23018285]
Clin Drug Investig. 2012 Nov;32(11):761-9.
BACKGROUND: The concentration of amyloid beta (Abeta) peptides in cerebrospinal fluid (CSF) is a biomarker for Alzheimer's disease (AD) pathology, and has been used to evaluate the effectiveness of gamma-secretase inhibition. Avagacestat is a selective gamma-secretase inhibitor in development for the treatment of AD. The primary objective of this study was to assess the effects of single oral doses of avagacestat on the CSF Abeta concentrations in healthy male subjects. Secondary objectives included single-dose pharmacokinetics in CSF and plasma, safety and tolerability. METHODS: This was a double-blind, placebo-controlled, randomized, single-dose study. Healthy male subjects were assigned to one of three sequential avagacestat dose panels (50, 200 and 400 mg) or placebo as single oral doses. RESULTS: 34 subjects were enrolled. Administration of a single dose of 200 or 400 mg of avagacestat resulted in a marked decrease in CSF Abeta(1-38), Abeta(1-40) and Abeta(1-42) concentrations vs placebo; with smaller decreases observed in the 50 mg dose group. Avagacestat was quickly absorbed into the systemic circulation, with a mean time to reach maximum plasma concentration (t(max)) of approximately 1-2 h, and a CSF t(max) of approximately 3 h. Adverse events were uncommon and occurred with similar frequency in the placebo and avagacestat groups. CONCLUSION: Avagacestat was safe, well tolerated, and resulted in a notable decrease in CSF Abeta concentrations, suggestive of gamma-secretase inhibition. The results warrant further clinical study in patients with AD.
Multicenter, randomized, double-blind, placebo-controlled, single-ascending dose study of the oral gamma-secretase inhibitor BMS-708163 (Avagacestat): tolerability profile, pharmacokinetic parameters, and pharmacodynamic markers.[Pubmed:22381714]
Clin Ther. 2012 Mar;34(3):654-67.
BACKGROUND: gamma-Secretase inhibitors (GSIs) are being investigated for their potential to modify the progression of Alzheimer disease based on their ability to regulate amyloid-beta (Abeta) accumulation. BMS-708163 (Avagacestat) is an oral GSI designed for selective inhibition of Abeta synthesis currently in development for the treatment of mild to moderate and predementia AD. In addition to the desired effect on Abeta synthesis, GSIs affect Notch processing, which is thought to mediate some toxic adverse effects reported with this drug class. Avagacestat produced up to 190-fold greater selectivity for Abeta synthesis than Notch processing in preclinical studies and may therefore produce less toxic adverse events than other less selective compounds. Presented here are the results of the first in-human study for this new GSI compound. OBJECTIVE: The goal of this study was to assess the tolerability profile, pharmacokinetic properties, and effects on pharmacodynamic markers (Abeta, trefoil factor family 3 protein, dual specificity phosphatase 6, and hairy and enhancer of split-1) of single, oral doses of avagacestat in healthy, young, male volunteers. METHODS: This was a multicenter, randomized, double-blind, placebo-controlled, single-ascending dose study in 8 healthy young men (age, 18-45 years) per dosing panel. Each study participant was randomized to receive a single dose of placebo (n = 2) or avagacestat (n = 6 for each dose) as an oral solution in 1 of 9 sequential dose panels (0.3, 1.5, 5, 15, 50, 100, 200, 400, and 800 mg). For determination of avagacestat, blood samples were obtained before dosing and for up to 144 hours after dosing. For participants in the 800-mg avagacestat dose panel, additional samples were obtained at 216, 312, and 648 hours. For 40-amino acid isoform of Abeta (Abeta(1-40)) assessment, plasma samples were collected before avagacestat administration and up to 72 hours after dosing. RESULTS: Avagacestat concentrations peaked quickly after oral administration and then had a biphasic decrease in concentrations with a prolonged terminal phase. Exposures were proportional with doses up to 200 mg. Avagacestat was well tolerated at single oral doses up to 800 mg, with a biphasic effect on plasma Abeta(1-40). Adverse events were predominately mild to moderate in severity with no evidence of dose dependence up to 200 mg. CONCLUSIONS: Results from this single-ascending dose study suggest that avagacestat was tolerated at a single-dose range of 0.3 to 800 mg and suitable for further clinical development.


