MK-0752γ-secretase inhibitor CAS# 471905-41-6 |

- BMS-708163 (Avagacestat)
Catalog No.:BCC2104
CAS No.:1146699-66-2
- DAPT (GSI-IX)
Catalog No.:BCC3618
CAS No.:208255-80-5
- YO-01027 (Dibenzazepine, DBZ)
Catalog No.:BCC2100
CAS No.:209984-56-5
- Flurizan
Catalog No.:BCC2342
CAS No.:51543-40-9
- Begacestat
Catalog No.:BCC2346
CAS No.:769169-27-9
Quality Control & MSDS
Number of papers citing our products

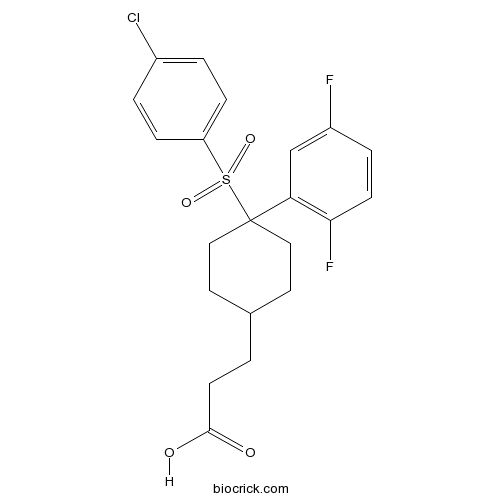
Chemical structure
3D structure
| Cas No. | 471905-41-6 | SDF | Download SDF |
| PubChem ID | 9803433 | Appearance | Powder |
| Formula | C21H21ClF2O4S | M.Wt | 442.9 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : ≥ 100 mg/mL (225.78 mM) Ethanol : 10 mg/mL (22.58 mM; Need ultrasonic) *"≥" means soluble, but saturation unknown. | ||
| Chemical Name | 3-[4-(4-chlorophenyl)sulfonyl-4-(2,5-difluorophenyl)cyclohexyl]propanoic acid | ||
| SMILES | C1CC(CCC1CCC(=O)O)(C2=C(C=CC(=C2)F)F)S(=O)(=O)C3=CC=C(C=C3)Cl | ||
| Standard InChIKey | XCGJIFAKUZNNOR-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C21H21ClF2O4S/c22-15-2-5-17(6-3-15)29(27,28)21(18-13-16(23)4-7-19(18)24)11-9-14(10-12-21)1-8-20(25)26/h2-7,13-14H,1,8-12H2,(H,25,26) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Targets | γ-secretase | ||||
| IC50 | 5 nM |
| Cell experiment: [1] | |
| Cell lines | SH-SY5Y cells |
| Preparation method | The solubility of this compound in DMSO is >10 mM. General tips for obtaining a higher concentration: Please warm the tube at 37 °C for 10 minutes and/or shake it in the ultrasonic bath for a while.Stock solution can be stored below -20°C for several months. |
| Reacting condition | IC50: 5 nM. |
| Applications | As a moderately potent γ-secretase inhibitor, MK-0752 inhibited the production of Aβ40 in a dose-dependent manner with an IC50 of 5 nM in human SH-SY5Y cells. |
| Animal experiment : [1] | |
| Animal models | Male CMP rhesus monkeys |
| Dosage form | Oral administration, 60 mg/kg and 240mg/kg |
| Application | Oral administration of MK-0752 demonstrated a dose-related reduction of Aβ levels. After 48 h, the Aβ levels with 240mg/kg treatment only recovered to 50%of baseline, while the 60mg/kg treatment group reached baseline at 30 h without overshoot. Plasma Aβ levels rebounded above baseline after MK-0752 inhibition (60 mg/kg, h 33–48). |
| Other notes | Please test the solubility of all compounds indoor, and the actual solubility may slightly differ with the theoretical value. This is caused by an experimental system error and it is normal. |
| References: [1] Cook J J, Wildsmith K R, Gilberto D B, et al. Acute γ-secretase inhibition of nonhuman primate CNS shifts amyloid precursor protein (APP) metabolism from amyloid-β production to alternative APP fragments without amyloid-β rebound[J]. The Journal of Neuroscience, 2010, 30(19): 6743-6750. | |

MK-0752 Dilution Calculator

MK-0752 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.2578 mL | 11.2892 mL | 22.5785 mL | 45.1569 mL | 56.4462 mL |
| 5 mM | 0.4516 mL | 2.2578 mL | 4.5157 mL | 9.0314 mL | 11.2892 mL |
| 10 mM | 0.2258 mL | 1.1289 mL | 2.2578 mL | 4.5157 mL | 5.6446 mL |
| 50 mM | 0.0452 mL | 0.2258 mL | 0.4516 mL | 0.9031 mL | 1.1289 mL |
| 100 mM | 0.0226 mL | 0.1129 mL | 0.2258 mL | 0.4516 mL | 0.5645 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||
Abstract
MK-0752, an inhibitor of γ-secretase, has been assessed for safety, maximum-tolerated dose, PKs, pharmacodynamics and antitumor efficacy in a phase I study.
Abstract
The MTD, DLTs and pharmacokinetic properties of MK-0752, a γ-secretase inhibitor, have been evaluated in children with refractory or recurrent CNS malignancies.
Abstract
The Cancer Research UK study CR0720-11 is briefly described in terms of objectives, methods and analysis procedures.
Abstract
The concentration of MK-0752, a γ-secretase inhibitor, in human plasma can be determined by the HTLC-ESI-MS/MS method, which was used to measure plasma MK-0752 levels in a Phase I study of pediatric patients with recurrent or refractory brain tumors.

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
MK-0752 is a potent gamma secretase inhibitor in clinical development (IC50 ~50 nM). Gamma secretase is an important component in the NOTCH cleavage machinery that catalyzes the cleavage of receptor protein substrates within their transmembrane domain. Inhibition of Notch inhibits BC cell proliferation in vitro. Notch signaling requires gamma secretase, which cleaves Notch, releasing the Notch intracellular domain (NICD) to activate transcription of target genes. NOTCH signaling plays an important role in normal tissue development, cell fate determination, proliferation, and survival. NOTCH signaling is activated following the binding of cognate ligands that include Delta1, Delta2, and Delta3 and Jagged1 and Jagged2.
Reference
I. E. Krop, M. Kosh, I. Fearen, J. Savoie, A. Dallob, C. Matthews, J. Stone, E. Winer, S. J. Freedman and P. Lorusso. Phase I pharmacokinetic (PK), and pharmacodynamic (PD) trial of the novel oral Notch inhibitor MK-0752 in patients (pts) with advanced breast cancer (BC) and other solid tumors. J Clin Oncol (Meeting Abstracts) June 2006 vol. 24 no. 18_suppl 10574.
Maryam Fouladi, Clinton F. Stewart, James Olson, Lars M. Wagner, Arzu Onar-Thomas, Mehmet Kocak, Roger J. Packer, Stewart Goldman, Sridharan Gururangan, Amar Gajjar, Tim Demuth, Larry E. Kun, James M. Boyett and Richard J. Gilbertson. Phase I Trial of MK-0752 in Children With Refractory CNS Malignancies: A Pediatric Brain Tumor Consortium Study. JCO September 10, 2011 vol. 29 no. 26 3529-3534
- Boc-D-Ser(Bzl)-OH
Catalog No.:BCC3448
CAS No.:47173-80-8
- (E)-Aldosecologanin
Catalog No.:BCN4631
CAS No.:471271-55-3
- Bufotaline
Catalog No.:BCN5368
CAS No.:471-95-4
- Stachydrine
Catalog No.:BCN8384
CAS No.:471-87-4
- (-)-Steviol
Catalog No.:BCN8358
CAS No.:471-80-7
- Dipterocarpol
Catalog No.:BCN5523
CAS No.:471-69-2
- alpha-Boswellic acid
Catalog No.:BCN5522
CAS No.:471-66-9
- Isocolumbin
Catalog No.:BCN5361
CAS No.:471-54-5
- Glycyrrhetinic acid
Catalog No.:BCN5942
CAS No.:471-53-4
- 8-Amino-7-oxononanoic acid
Catalog No.:BCN1778
CAS No.:4707-58-8
- Atraric acid
Catalog No.:BCN5521
CAS No.:4707-47-5
- alpha-Lapachone
Catalog No.:BCN5520
CAS No.:4707-33-9
- Ruscogenin
Catalog No.:BCN6287
CAS No.:472-11-7
- Betulinic acid
Catalog No.:BCN5524
CAS No.:472-15-1
- Telocinobufagin
Catalog No.:BCN2359
CAS No.:472-26-4
- Butyrospermol
Catalog No.:BCN3340
CAS No.:472-28-6
- Masticadienolic acid
Catalog No.:BCN5525
CAS No.:472-30-0
- Astaxanthin
Catalog No.:BCN2248
CAS No.:472-61-7
- H-Ser(Bzl)-OH
Catalog No.:BCC3031
CAS No.:4726-96-9
- Kartogenin
Catalog No.:BCC6211
CAS No.:4727-31-5
- 1-Benzyl-4-hydroxypiperidine
Catalog No.:BCC8459
CAS No.:4727-72-4
- 8(14),15-Isopimaradien-3-ol
Catalog No.:BCN5526
CAS No.:4728-30-7
- SB 366791
Catalog No.:BCC7128
CAS No.:472981-92-3
- alpha-Cyperone
Catalog No.:BCN1193
CAS No.:473-08-5
Sequential combination therapy of ovarian cancer with cisplatin and gamma-secretase inhibitor MK-0752.[Pubmed:26704638]
Gynecol Oncol. 2016 Mar;140(3):537-44.
OBJECTIVE: Ovarian cancer is one of the most lethal of women cancers and lack potent therapeutic options. There have many evidences demonstrate the Notch signaling has deregulation in variety of human malignancies.MK-0752 is a novel potent gamma-secretase inhibitor and now assessed in clinical trial for treatment of several types of cancer, our objective was to investigate the anticancer effects and mechanisms of MK-0752 alone or combined with cisplatin in ovarian cancer. METHODS: Cell lines used: A2780, OVCAR3, SKOV3, HO8910PM, the effects of MK-0752 and cisplatin on cell proliferation were measured by MTT assay. The effect of combination treatment was examined by isobologram analysis. The distribution of cell cycle and cell apoptosis were analyzed using PI and Annexin V-FITC/PI staining by flow cytometric analysis. The mechanism in biochemistry was analyzed by using Western blot. Mouse xenograft model of A2780 was established to observe the anti-ovarian cancer effects in vivo setting, nude mice were randomized into four groups (n=6 per group) and treated every 4 days with control (solvent) group, MK-0752(25mg/kg) group, cisplatin (2mg/kg)group, combination group (both of MK-0752 and cisplatin). RESULTS: MK-0752 alone actively induced cell growth inhibition, G2/M phase cell cycle arrest and apoptosis with down-regulation of Notch1 and its downstream effectors including Hes1, XIAP, c-Myc and MDM2 in a dose- and time-dependent manner. Moreover, sequential combination of cisplatin prior to MK-0752 significantly promoted cell apoptosis and inhibited the subcutaneous xenograft growth of ovarian cancer in nude mice. CONCLUSION: Our data supports the sequential combination of cisplatin prior to MK-0752 is a highly promising novel experimental therapeutic strategy against ovarian cancer.
A parallel-arm phase I trial of the humanised anti-IGF-1R antibody dalotuzumab in combination with the AKT inhibitor MK-2206, the mTOR inhibitor ridaforolimus, or the NOTCH inhibitor MK-0752, in patients with advanced solid tumours.[Pubmed:25290091]
Br J Cancer. 2014 Nov 11;111(10):1932-44.
BACKGROUND: Two strategies to interrogate the insulin growth factor 1 receptor (IGF-1R) pathway were investigated: vertical inhibition with dalotuzumab and MK-2206 or ridaforolimus to potentiate PI3K pathway targeting and horizontal cross-talk inhibition with dalotuzumab and MK-0752 to exert effects against cellular proliferation, angiogenesis, and stem cell propagation. METHODS: A phase I, multi-cohort dose escalation study was conducted in patients with advanced solid tumours. Patients received dalotuzumab (10 mg kg(-1)) and escalating doses of MK-2206 (90-200 mg) or escalating doses of dalotuzumab (7.5-10 mg kg(-1)) and MK-0752 (1800 mg) weekly. Upon maximum tolerated dose determination, patients with low-RAS signature, high-IGF1 expression ovarian cancer were randomised to dalotuzumab/MK-2206 versus dalotuzumab/ridaforolimus, whereas patients with high IGF1/low IGF2 expression colorectal cancer received dalotuzumab/MK-0752. RESULTS: A total of 47 patients were enrolled: 29 in part A (18 in the dalotuzumab/MK-2206 arm and 11 in the dalotuzumab/MK-0752 arm) and 18 in part B (6 in each arm). Dose-limiting toxicities (DLTs) for dalotuzumab/MK-2206 included grade 4 neutropenia and grade 3 serum sickness-like reaction, maculopapular rash, and gastrointestinal inflammation. For dalotuzumab/MK-0752, DLTs included grade 3 dehydration, rash, and diarrhoea. Seven patients remained on study for >4 cycles. CONCLUSIONS: Dalotuzumab/MK-2206 and dalotuzumab/MK-0752 combinations were tolerable. Further developments of prospectively validated predictive biomarkers to aid in patient selection for anti-IGF-1R therapies are needed.
Phase I trial of weekly MK-0752 in children with refractory central nervous system malignancies: a pediatric brain tumor consortium study.[Pubmed:25930724]
Childs Nerv Syst. 2015 Aug;31(8):1283-9.
PURPOSE: Amplification and high levels of NOTCH ligand expression have been identified in several types of pediatric brain tumors. A phase I trial of weekly MK-0752, an oral inhibitor of gamma-secretase, was conducted in children with recurrent central nervous system (CNS) malignancies to estimate the maximum tolerated dose, dose-limiting toxicities (DLT), pharmacokinetics (PK), and pharmacodynamics of weekly MK-0752. METHODS: MK-0752 was administered once weekly at 1000 and 1400 mg/m(2) using a rolling-6 design. PK analysis was performed during the first course. NOTCH and HES expression was assessed by immunohistochemistry and Western blot. RESULTS: Ten eligible patients were enrolled (median age 8.8 years; range 3.1-19.2) with diagnoses of brain stem glioma (n = 3), ependymoma (n = 2), anaplastic astrocytoma (n = 1), choroid plexus carcinoma (n = 2), medulloblastoma (n = 1), and primitive neuroectodermal tumor (n = 1). Nine were evaluable for toxicity. One DLT of fatigue occurred in the six evaluable patients enrolled at 1000 mg/m(2)/dose. No DLTs were experienced by three patients treated at 1400 mg/m(2)/dose. Non-dose-limiting grade 3 toxicities included lymphopenia, neutropenia, and anemia. Median number of treatment courses was 2 (range 1-10). Two patients continued on therapy for at least 6 months. The median (range) C(max) of MK-0752 was 88.2 mug/mL (40.6 to 109 mug/mL) and 60.3 mug/mL (59.2 to 91.9 mug/mL) in patients receiving 1000 and 1400 mg/m(2)/week, respectively. NOTCH expression was decreased in six of seven patients for whom tissue was available at 24 h post-MK-0752. CONCLUSION: MK-0752 is well tolerated and exhibits target inhibition at 1000 and 1400 mg/m(2)/week in children with recurrent CNS malignancies.
Results of a phase 1 trial combining ridaforolimus and MK-0752 in patients with advanced solid tumours.[Pubmed:26199039]
Eur J Cancer. 2015 Sep;51(14):1865-73.
BACKGROUND: The phosphatidylinositol 3-kinase/protein kinase-B/mammalian target of rapamycin (PI3K-AKT-mTOR) signalling pathway is aberrantly activated in several cancers. Notch signalling maintains cell proliferation, growth and metabolism in part by driving the PI3K pathway. Combining the mTOR inhibitor ridaforolimus with the Notch inhibitor MK-0752 may increase blockade of the PI3K pathway. METHODS: This phase I dose-escalation study (NCT01295632) aimed to define the dose-limiting toxicities (DLTs) and maximum tolerated dose (MTD) of combination oral ridaforolimus (rising doses starting at 20 mg, 5 days/week) and oral MK-0752 (1800 mg once weekly) in patients with solid tumours. No intrapatient dose escalation was permitted. RESULTS: Twenty eight patients were treated on study. Ridaforolimus doses were escalated from 20 to 30 mg/day. Among 14 evaluable patients receiving ridaforolimus 20 mg, one DLT (grade 2 stomatitis, second episode) was reported. Among eight evaluable patients receiving ridaforolimus 30 mg, three DLTs were reported (one each grade 3 stomatitis, grade 3 diarrhoea, and grade 3 asthenia). The MTD was 20 mg daily ridaforolimus 5 days/week+1800 mg weekly MK-0752. The most common drug-related adverse events included stomatitis, diarrhoea, decreased appetite, hyperglycaemia, thrombocytopenia, asthenia and rash. Two of 15 (13%) patients with head and neck squamous cell carcinoma (HNSCC) had responses: one with complete response and one with partial response. In addition, one patient experienced stable disease 6 months. CONCLUSIONS: Combined ridaforolimus and MK-0752 showed activity in HNSCC. However, a high number of adverse events were reported at the MTD, which would require careful management during future clinical development.


