Ibuprofeninhibitor of cyclooxygenase 1 and cyclooxygenase 2 CAS# 15687-27-1 |
- FK 3311
Catalog No.:BCC1576
CAS No.:116686-15-8
- Iguratimod
Catalog No.:BCC1641
CAS No.:123663-49-0
- Celecoxib
Catalog No.:BCC1099
CAS No.:169590-42-5
- Etoricoxib
Catalog No.:BCC1565
CAS No.:202409-33-4
- Ibuprofen Lysine
Catalog No.:BCC2547
CAS No.:57469-77-9
Quality Control & MSDS
Number of papers citing our products

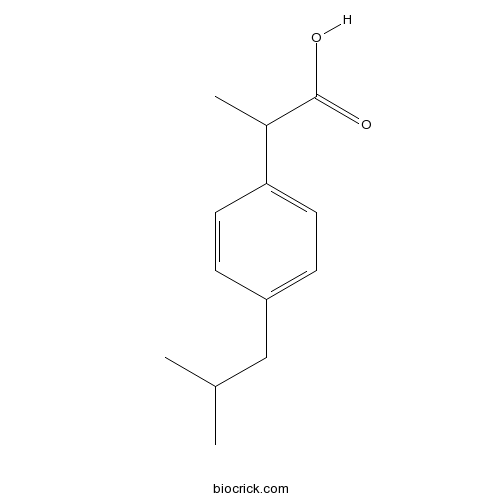
Chemical structure

3D structure
| Cas No. | 15687-27-1 | SDF | Download SDF |
| PubChem ID | 3672 | Appearance | Powder |
| Formula | C13H18O2 | M.Wt | 206.28 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | (±)-Ibuprofe | ||
| Solubility | DMSO : ≥ 100 mg/mL (484.78 mM) H2O : < 0.1 mg/mL (insoluble) *"≥" means soluble, but saturation unknown. | ||
| Chemical Name | 2-[4-(2-methylpropyl)phenyl]propanoic acid | ||
| SMILES | CC(C)CC1=CC=C(C=C1)C(C)C(=O)O | ||
| Standard InChIKey | HEFNNWSXXWATRW-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C13H18O2/c1-9(2)8-11-4-6-12(7-5-11)10(3)13(14)15/h4-7,9-10H,8H2,1-3H3,(H,14,15) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Ibuprofen is an anti-inflammatory inhibitor targeting COX-1 and COX-2 with IC50 of 13 μM and 370 μM, respectively.In Vitro:Ibuprofen inhibits the enzyme cyclooxygenase COX-1 and COX-2, which convert arachidonic acid to prostaglandin H2 (PGH2). Its action is similar to aspirin, indomethacin and all other NSAIDs in intact cells, broken cells, and purified enzyme preparations[1]. Ibuprofen inhibits the constitutive activation of NF-κB and IKKα in the androgen-independent prostate tumor cells PC-3 and DU-145. It sensitizes prostate cells to ionizing radiation and blocks stimulated activation of NF-κB following exposure to TNFα or ionizing radiation in the androgen-sensitive prostate tumor cell line LNCaP. Both of these cannot be attributed directly to inhibition of IκB-α kinase but to inhibition of an upstream regulator of IKKα[2]. Ibuprofen exerts an anticancer effect by reducing survival of cancer cells. Ibuprofen is more efficacious than aspirin and acetaminophen, and comparable with (R)-flurbiprofen and indomethacin in induction of p75NTR protein expression in cell lines from bladder and other organs[3].In Vivo:Ibuprofen reacts with the heme group of cyclooxygenase to prevent arachidonic acid conversion. Prior exposure to Ibuprofen in vivo protects cyclooxygenase completely from the irreversible effects of aspirin in platelets[4]. Ibuprofen treatment is effective in attenuating joint inflammation and early articular cartilage degeneration in the adult female Sprague-Dawley rat model induced by high-repetition and high-force (HRHF) task. It dose this by blocking the increases in serum C1 and 2C (a biomarker of collagen I and II degradation) as well as the ratio of collagen degradation to synthesis (C1, 2C/CPII, the latter a biomarker of collage type II synthesis) induced by HRHF[5]. References: | |||||

Ibuprofen Dilution Calculator

Ibuprofen Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 4.8478 mL | 24.2389 mL | 48.4778 mL | 96.9556 mL | 121.1945 mL |
| 5 mM | 0.9696 mL | 4.8478 mL | 9.6956 mL | 19.3911 mL | 24.2389 mL |
| 10 mM | 0.4848 mL | 2.4239 mL | 4.8478 mL | 9.6956 mL | 12.1194 mL |
| 50 mM | 0.097 mL | 0.4848 mL | 0.9696 mL | 1.9391 mL | 2.4239 mL |
| 100 mM | 0.0485 mL | 0.2424 mL | 0.4848 mL | 0.9696 mL | 1.2119 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Ibuprofen is an inhibitor of cyclooxygenase 1 and cyclooxygenase 2 with IC50 values of 12 and 80 μM, respectively [1].
Cyclooxygenase (COX) is an enzyme that is responsible for the formation of prostaglandins, prostacyclin and thromboxane.
In HCT-116 p53wt or HCT-116 p53-/-colon carcinoma cell lines, S- and R-ibuprofen induced apoptosis and blocked cell cycle is in part dependent on p53. The anti-proliferative effects were significantly higher in the p53wt cell line than in the p53-deficient cells [2].
In nude mice model bearing HCT-116 p53wt and p53-/- xenografts, R-ibuprofen significantly inhibited the growth of p53wt expressing xenografts and only a small inhibition of p53-/- xenografts [2]. In hypercholesterolemic animals, ibuprofen reduced the levels of total cholesterol, VLDL, LDL, triglycerides and atherogenic index. Also, ibuprofen inhibited COX enzymes and inhibited the generation of free radicals during prostaglandins synthesis, which reduced the levels of lipid peroxidation, superoxide dismutase [3]. In rats, ibuprofen (60 mg/kg) improved mechanical hyperalgesia through reducing central hyperexcitability [4].
References:
[1]. Kato M, Nishida S, Kitasato H, et al. Cyclooxygenase-1 and cyclooxygenase-2 selectivity of non-steroidal anti-inflammatory drugs: investigation using human peripheral monocytes. J Pharm Pharmacol, 2001, 53(12): 1679-1685.
[2]. Janssen A, Schiffmann S, Birod K, et al. p53 is important for the anti-proliferative effect of ibuprofen in colon carcinoma cells. Biochem Biophys Res Commun, 2008, 365(4): 698-703.
[3]. Dabhi JK, Solanki JK, Mehta A. Antiatherosclerotic activity of ibuprofen, a non-selective COX inhibitor--an animal study. Indian J Exp Biol, 2008, 46(6): 476-481.
[4]. Redondo-Castro E, Navarro X. Chronic ibuprofen administration reduces neuropathic pain but does not exert neuroprotection after spinal cord injury in adult rats. Exp Neurol, 2014, 252: 95-103.
- Cephalexin
Catalog No.:BCC4646
CAS No.:15686-71-2
- Hosenkoside A
Catalog No.:BCN4962
CAS No.:156791-82-1
- Iristectorene B
Catalog No.:BCN7695
CAS No.:156791-81-0
- Agroastragaloside I
Catalog No.:BCC8294
CAS No.:156769-94-7
- 3-beta-Hydroxyergost-5-en-7-one
Catalog No.:BCN1704
CAS No.:156767-69-0
- Hosenkoside C
Catalog No.:BCN2632
CAS No.:156764-83-9
- Hosenkoside B
Catalog No.:BCN4584
CAS No.:156764-82-8
- (RS)-(±)-Sulpiride
Catalog No.:BCC6835
CAS No.:15676-16-1
- Delphinidin-3-O-rutinoside chloride
Catalog No.:BCN3115
CAS No.:15674-58-5
- SNC 80
Catalog No.:BCC6785
CAS No.:156727-74-1
- Rostafuroxin (PST 2238)
Catalog No.:BCC6431
CAS No.:156722-18-8
- Org 20599
Catalog No.:BCC7470
CAS No.:156685-94-8
- N1,N11-Diethylnorspermine tetrahydrochloride
Catalog No.:BCC6654
CAS No.:156886-85-0
- Licofelone
Catalog No.:BCC4432
CAS No.:156897-06-2
- OSU 6162 hydrochloride
Catalog No.:BCC7424
CAS No.:156907-84-5
- Isosalicifolin
Catalog No.:BCN6502
CAS No.:156974-99-1
- Grosvenorin
Catalog No.:BCN1262
CAS No.:156980-60-8
- Wedeliatrilolactone A
Catalog No.:BCN6732
CAS No.:156993-29-2
- H-D-Arg-OH
Catalog No.:BCC2868
CAS No.:157-06-2
- 5,7-Dihydroxy-3,4',8-trimethoxyflavone
Catalog No.:BCN1551
CAS No.:1570-09-8
- U 89843A
Catalog No.:BCC7466
CAS No.:157013-85-9
- SNAP 5089
Catalog No.:BCC7350
CAS No.:157066-77-8
- Lipedoside B-III
Catalog No.:BCC8201
CAS No.:157085-48-8
- 3-Amino-4-hydroxybenzoic acid
Catalog No.:BCC8610
CAS No.:1571-72-8
The Effect of Ibuprofen on Cytokine Production by Mononuclear Cells from Schizophrenic Patients.[Pubmed:28374670]
Folia Biol (Praha). 2017;63(1):13-19.
The existence of a restrained inflammatory state in schizophrenic individuals posed the question whether anti-inflammatory drugs may exert antipsychotic effects. Therefore, the effect of Ibuprofen (IB) on cytokine production by human peripheral blood mononuclear cells (PBMC) from schizophrenic patients was examined and compared to that of healthy subjects. PBMC from 25 schizophrenic patients and 24 healthy volunteers were incubated for 24 h with lipopolysaccharide (LPS) in the absence or presence of various concentrations of IB. The levels of IL-1beta, IL-6, TNF-alpha, IL-10 and IL-1ra in the supernatants were tested applying ELISA kits. The secretion of TNF-alpha by cells from schizophrenic patients was significantly lower compared with controls. IB caused stimulation of TNF-alpha and IL-6 production by cells of the two groups and enhanced IL-1beta secretion by cells from schizophrenic patients. IB inhibited IL-1ra and IL-10 generation by cells from the two groups. Without IB, IL-1ra secretion was negatively correlated with the disease severity, while 200 mug/ml of IB positively correlated with the PANSS total score. IL-10 production was positively correlated with the PANSS positive subscale score both in the absence or presence of IB. The findings suggest that the effect of IB on the production of inflammatory cytokines may benefit the health of schizophrenic patients.
Significant solubility of carbon dioxide in Soluplus((R)) facilitates impregnation of ibuprofen using supercritical fluid technology.[Pubmed:28375669]
Pharm Dev Technol. 2018 Sep;23(7):697-705.
Treatment of Soluplus((R)) with supercritical carbon dioxide allows promising applications in preparing dispersions of amorphous solids. Several characterization techniques were employed to reveal this effect, including CO2 gas sorption under high pressure and physicochemical characterizations techniques. A gravimetric method was used to determine the solubility of carbon dioxide in the polymer at elevated pressure. The following physicochemical characterizations were used: thermal analysis, X-ray diffraction, Fourier transform, infrared spectroscopy and scanning electron microscopy. Drug loading of the polymer with Ibuprofen as a model drug was also investigated. The proposed treatment with supercritical carbon dioxide allows to prepare solid solutions of Soluplus((R)) in less than two hours at temperatures that do not exceed 45 degrees C, which is a great advantage to be used for thermolabile drugs. The advantages of using this technology for Soluplus((R)) formulations lies behind the high sorption capability of carbon dioxide inside the polymer. This will ensure rapid diffusion of the dissolved/dispersed drug inside the polymer under process conditions and rapid precipitation of the drug in the amorphous form during depressurization accompanied by foaming of the polymer.
Encapsulation of Ibuprofen in CD-MOF and Related Bioavailability Studies.[Pubmed:28355489]
Mol Pharm. 2017 May 1;14(5):1831-1839.
Although Ibuprofen is one of the most widely used nonsteroidal anti-inflammatory drugs (NSAIDs), it exhibits poor solubility in aqueous and physiological environments as a free acid. In order to improve its oral bioavailability and rate of uptake, extensive research into the development of new formulations of Ibuprofen has been undertaken, including the use of excipients as well as Ibuprofen salts, such as Ibuprofen lysinate and Ibuprofen, sodium salt. The ultimate goals of these studies are to reduce the time required for maximum uptake of Ibuprofen, as this period of time is directly proportional to the rate of onset of analgesic/anti-inflammatory effects, and to increase the half-life of the drug within the body; that is, the duration of action of the effects of the drug. Herein, we present a pharmaceutical cocrystal of Ibuprofen and the biocompatible metal-organic framework called CD-MOF. This metal-organic framework (MOF) is based upon gamma-cyclodextrin (gamma-CD) tori that are coordinated to alkali metal cations (e.g., K(+) ions) on both their primary and secondary faces in an alternating manner to form a porous framework built up from (gamma-CD)6 cubes. We show that Ibuprofen can be incorporated within CD-MOF-1 either by (i) a crystallization process using the potassium salt of Ibuprofen as the alkali cation source for production of the MOF or by (ii) absorption and deprotonation of the free-acid, leading to an uptake of 23-26 wt % of Ibuprofen within the CD-MOF. In vitro viability studies revealed that the CD-MOF is inherently not affecting the viability of the cells with no IC50 value determined up to a concentration of 100 muM. Bioavailability investigations were conducted on mice, and the Ibuprofen/CD-MOF pharmaceutical cocrystal was compared to control samples of the potassium salt of Ibuprofen in the presence and absence of gamma-CD. From these animal studies, we observed that the Ibuprofen/CD-MOF-1 cocrystal exhibits the same rapid uptake of Ibuprofen as the Ibuprofen potassium salt control sample with a peak plasma concentration observed within 20 min, and the cocrystal has the added benefit of a 100% longer half-life in blood plasma samples and is intrinsically less hygroscopic than the pure salt form.


