CelecoxibSelective cyclooxygenase-2 (COX-2) inhibitor CAS# 169590-42-5 |
- FK 3311
Catalog No.:BCC1576
CAS No.:116686-15-8
- Iguratimod
Catalog No.:BCC1641
CAS No.:123663-49-0
- Ibuprofen
Catalog No.:BCC3791
CAS No.:15687-27-1
- Etoricoxib
Catalog No.:BCC1565
CAS No.:202409-33-4
- Ibuprofen Lysine
Catalog No.:BCC2547
CAS No.:57469-77-9
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 169590-42-5 | SDF | Download SDF |
| PubChem ID | 2662 | Appearance | Powder |
| Formula | C17H14F3N3O2S | M.Wt | 381.37 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | SC58635, Celebrex;SC 58635;SC-58635 | ||
| Solubility | DMSO : ≥ 50 mg/mL (131.11 mM) *"≥" means soluble, but saturation unknown. | ||
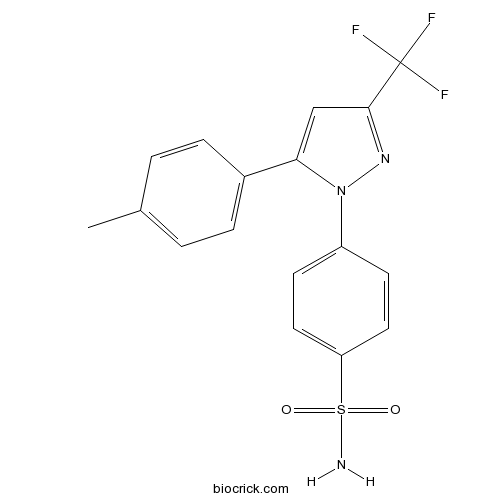
| Chemical Name | 4-[5-(4-methylphenyl)-3-(trifluoromethyl)pyrazol-1-yl]benzenesulfonamide | ||
| SMILES | CC1=CC=C(C=C1)C2=CC(=NN2C3=CC=C(C=C3)S(=O)(=O)N)C(F)(F)F | ||
| Standard InChIKey | RZEKVGVHFLEQIL-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C17H14F3N3O2S/c1-11-2-4-12(5-3-11)15-10-16(17(18,19)20)22-23(15)13-6-8-14(9-7-13)26(21,24)25/h2-10H,1H3,(H2,21,24,25) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Selective cyclooxygenase-2 (COX-2) inhibitor (IC50 values are 15 and 0.04 μM for COX-1 and COX-2 respectively). Anti-inflammatory with shorter plasma half-life in vivo than SC 58121. Displays chemopreventive activity in in vivo tumor models. |

Celecoxib Dilution Calculator

Celecoxib Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.6221 mL | 13.1106 mL | 26.2213 mL | 52.4425 mL | 65.5531 mL |
| 5 mM | 0.5244 mL | 2.6221 mL | 5.2443 mL | 10.4885 mL | 13.1106 mL |
| 10 mM | 0.2622 mL | 1.3111 mL | 2.6221 mL | 5.2443 mL | 6.5553 mL |
| 50 mM | 0.0524 mL | 0.2622 mL | 0.5244 mL | 1.0489 mL | 1.3111 mL |
| 100 mM | 0.0262 mL | 0.1311 mL | 0.2622 mL | 0.5244 mL | 0.6555 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Celecoxib is a highly selective inhibitor of cyclooxygenase-2 (COX-2) with IC50 value of 40nM [1].
In vitro, celecoxib not only reduced the production of PGE2 but also inhibited the downstream effects of PGE2. Celecoxib blocked migration and invasion of A549 cells increased by PGE2 in the wound healing and transwell assays. Additionally, celecoxib reduced MMP9 mRNA expression which was increased by PGE2. Moreover, celecoxib enhanced E-cadherin mRNA expression which was inhibited by PGE2 [2].
In vivo, celecoxib inhibited the increase in metastases of A549 cells and significantly reduced the increase in PGE2 plasma level in mice receiving unilateral pneumonectomy [2].
References:
[1] Penning TD1, Talley JJ, Bertenshaw SR, Carter JS, Collins PW, Docter S, Graneto MJ, Lee LF, Malecha JW, Miyashiro JM, Rogers RS, Rogier DJ, Yu SS,AndersonGD, Burton EG, Cogburn JN, Gregory SA, Koboldt CM, Perkins WE, Seibert K, Veenhuizen AW, Zhang YY, Isakson PC. Synthesis and biological evaluation of the 1,5-diarylpyrazole class of cyclooxygenase-2 inhibitors: identification of 4-[5-(4-methylphenyl)-3-(trifluoromethyl)-1H-pyrazol-1-yl]benze nesulfonamide (SC-58635, celecoxib). J Med Chem. 1997 Apr 25;40(9):1347-65.
[2] Zhang S1, Da L1, Yang X1, Feng D1, Yin R1, Li M1, Zhang Z1, Jiang F2, Xu L3. Celecoxib potentially inhibits metastasis of lung cancer promoted by surgery in mice, via suppression of the PGE2-modulated β-catenin pathway.
Toxicol Lett. 2014 Mar 3;225(2):201-7.
- Deracoxib
Catalog No.:BCC4108
CAS No.:169590-41-4
- Floricaline
Catalog No.:BCN2104
CAS No.:16958-32-0
- Floridanine
Catalog No.:BCN2103
CAS No.:16958-31-9
- Florosenine
Catalog No.:BCN2108
CAS No.:16958-30-8
- Otosenine
Catalog No.:BCN2107
CAS No.:16958-29-5
- Fmoc-Ala(4-pyridyl)-OH
Catalog No.:BCC3327
CAS No.:169555-95-7
- IRL-2500
Catalog No.:BCC7192
CAS No.:169545-27-1
- Protostemotinine
Catalog No.:BCN8314
CAS No.:169534-85-4
- RS 17053 hydrochloride
Catalog No.:BCC6874
CAS No.:169505-93-5
- Boc-N-Me-Ala-OH
Catalog No.:BCC3209
CAS No.:16948-16-6
- 3β,7β,15β-trihydroxy-11-oxo-lanosta-8-en-24->20 lactone
Catalog No.:BCC8643
CAS No.:1694587-15-9
- Boc-D-Leu-OH.H2O
Catalog No.:BCC3409
CAS No.:16937-99-8
- Odoratone
Catalog No.:BCN1105
CAS No.:16962-90-6
- Trichosanatine
Catalog No.:BCN1818
CAS No.:169626-16-8
- Isodonal
Catalog No.:BCN3390
CAS No.:16964-56-0
- Z-Thr(tBu)-OH.DCHA
Catalog No.:BCC2565
CAS No.:16966-07-7
- Ro 60-0175 fumarate
Catalog No.:BCC7196
CAS No.:169675-09-6
- Angelol K
Catalog No.:BCN8142
CAS No.:169736-93-0
- Dibutyryl-cAMP, sodium salt
Catalog No.:BCC8079
CAS No.:16980-89-5
- Mesuol
Catalog No.:BCN6583
CAS No.:16981-20-7
- Iso-cuparenal
Catalog No.:BCN7350
CAS No.:16982-01-7
- LY 333531 hydrochloride
Catalog No.:BCC7969
CAS No.:169939-93-9
- Reserpine hydrochloride
Catalog No.:BCC4279
CAS No.:16994-56-2
- Bufarenogin
Catalog No.:BCN2297
CAS No.:17008-65-0
Adding Celecoxib With or Without Zoledronic Acid for Hormone-Naive Prostate Cancer: Long-Term Survival Results From an Adaptive, Multiarm, Multistage, Platform, Randomized Controlled Trial.[Pubmed:28300506]
J Clin Oncol. 2017 May 10;35(14):1530-1541.
Purpose Systemic Therapy for Advanced or Metastatic Prostate Cancer: Evaluation of Drug Efficacy is a randomized controlled trial using a multiarm, multistage, platform design. It recruits men with high-risk, locally advanced or metastatic prostate cancer who were initiating long-term hormone therapy. We report survival data for two Celecoxib (Cel)-containing comparisons, which stopped accrual early at interim analysis on the basis of failure-free survival. Patients and Methods Standard of care (SOC) was hormone therapy continuously (metastatic) or for >/= 2 years (nonmetastatic); prostate (+/- pelvic node) radiotherapy was encouraged for men without metastases. Cel 400 mg was administered twice a day for 1 year. Zoledronic acid (ZA) 4 mg was administered for six 3-weekly cycles, then 4-weekly for 2 years. Stratified random assignment allocated patients 2:1:1 to SOC (control), SOC + Cel, or SOC + ZA + Cel. The primary outcome measure was all-cause mortality. Results were analyzed with Cox proportional hazards and flexible parametric models adjusted for stratification factors. Results A total of 1,245 men were randomly assigned (Oct 2005 to April 2011). Groups were balanced: median age, 65 years; 61% metastatic, 14% N+/X M0, 25% N0M0; 94% newly diagnosed; median prostate-specific antigen, 66 ng/mL. Median follow-up was 69 months. Grade 3 to 5 adverse events were seen in 36% SOC-only, 33% SOC + Cel, and 32% SOC + ZA + Cel patients. There were 303 control arm deaths (83% prostate cancer), and median survival was 66 months. Compared with SOC, the adjusted hazard ratio was 0.98 (95% CI, 0.80 to 1.20; P = .847; median survival, 70 months) for SOC + Cel and 0.86 (95% CI, 0.70 to 1.05; P =.130; median survival, 76 months) for SOC + ZA + Cel. Preplanned subgroup analyses in men with metastatic disease showed a hazard ratio of 0.78 (95% CI, 0.62 to 0.98; P = .033) for SOC + ZA + Cel. Conclusion These data show no overall evidence of improved survival with Cel. Preplanned subgroup analyses provide hypotheses for future studies.
A stratified randomized double-blind phase II trial of celecoxib for treating patients with cervical intraepithelial neoplasia: The potential predictive value of VEGF serum levels: An NRG Oncology/Gynecologic Oncology Group study.[Pubmed:28285845]
Gynecol Oncol. 2017 May;145(2):291-297.
PURPOSE: To examine the effect of Celecoxib on cervical intraepithelial neoplasia 3 (CIN 3). This is a NRG Oncology/Gynecologic Oncology Group study with translational biomarkers. PATIENTS AND METHODS: Patients with CIN 3 were randomized to Celecoxib 400mg once daily (67 patients) or placebo (63 patients) for 14-18weeks. The primary outcome measure was histologic regression. A test of equal probabilities of success between two therapies was conducted, using Fisher's Exact Test at alpha=10% and 90% power when the treatment arm boosted the probability of success by 30%. Translational analysis included cervical tissue HPV genotyping, COX-2 expression in biopsies, and serum Celecoxib and VEGF levels. RESULTS: In primary analysis, histologic regression was not significantly higher in the Celecoxib group (40%) than in the placebo group (34.1%). However, exploratory analyses suggest patients with high serum VEGF levels exhibited greater regression in the Celecoxib arm (47.3%) than in the placebo arm (14.3%). Regression rates were similar by treatment group in patients with low VEGF. VEGF levels increased over time in the placebo group, but remained the same in the treatment group. COX-2 expression in cervical biopsies declined from pre-treatment to the end of treatment with Celecoxib; it did not change with placebo. CONCLUSIONS: Celecoxib at 400mg once daily for 14-18weeks did not significantly decrease the severity of CIN 3 compared with placebo except, possibly, in subjects with high baseline VEGF. Therefore, serum VEGF levels might identify patients who may benefit from Celecoxib or other therapies, personalizing future chemoprevention trials for CIN 3.
Celecoxib-erlotinib combination treatment enhances radiosensitivity in A549 human lung cancer cell.[Pubmed:28282799]
Cancer Biomark. 2017;19(1):45-50.
BACKGROUND: Radiosensitivity by blocking the epidermal growth factor receptor and cyclooxygenase-2 pathways with erlotinib and Celecoxib in A549 human lung cancer cell was investigated. METHODS: MTT assays were used to detect the antitumor effects of erlotinib and Celecoxib in A549 cells. Colony formation assays were used to evaluate the antitumor effects. Flow cytometry analysis was used to assess the cell cycle and cell apoptosis, and western blotting analysis was performed to evaluate the expression of AKT and phosphorylated AKT. RESULTS: Either erlotinib or Celecoxib inhibited the A549 cell proliferation in a dose-dependent manner. Combining Erlotinib or Celecoxib with radiation can suppress the cell colony formation and the Dq, D0, SF2 of the combining erlotinib or Celecoxib with radiation was lower than in the combinations either erlotinib or Celecoxib with radiation (t= 6.62, P< 0.05). The SER of radiation with Celecoxib or erlotinib and Celecoxib and erlotinib were 1.299, 1.503 and 2.217, respectively. The Flow cytometry analysis results showed that either Celecoxib or erlotinib could induce G0/G1 arrest, and reduction of S phase cell proportion, especially when combinations erlotinib-Celecoxib with radiation. Either Celecoxib or erlotinib could enhance radiation-induced apoptosis, especially significant when combinations erlotinib-Celecoxib with radiation. Moreover, radiation can promote the expression of pAKT, and the pAKT was remarkably lowest in the combinations erlotinib-Celecoxib with radiation group (t= 4.89, P< 0.05). CONCLUSIONS: Blocking both EGFR- and COX-2-related pathways could enhance the antitumor effect of radiation. The underlying mechanisms including the enhancement of apoptosis and radiation-induced G0/G1 arrest, possibly via inhibiting the PI3K/AKT signaling pathway.
Isobolographic Analyses of Proglumide-Celecoxib Interaction in Rats with Painful Diabetic Neuropathy.[Pubmed:28370133]
Drug Dev Res. 2017 Mar;78(2):116-123.
Preclinical Research The aim of the present study was to analyze the antihyperalgesic and antiallodynic interaction between the non-selective cholecystokinin (CCK) antagonist receptor, proglumide, and the selective cyclooxygenase-2 inhibitor, Celecoxib in streptozotocin (STZ)-induced diabetic rats. Hyperalgesia was evaluated in the formalin test and tactile allodynia using von Frey filaments. Isobolographic analyses were employed to define the nature of the compound interactions, using a fixed dose ratio (0.5:0.5). Proglumide (20-160 mg/kg) and Celecoxib (0.3-30 mg/kg) in these fixed dose ratio combinations induced dose-dependent antihyperalgesia and an antiallodynic effect in diabetic rats. ED40 values were calculated for the treatments and an isobologram was constructed. Theoretical ED40 values for combination proglumide-Celecoxib estimated from the isobolograms for antihyperalgesic and antiallodynic activity (30.50 +/- 1.90 mg/kg and 45.81 +/- 4.55 mg/kg, respectively) were obtained, while experimental ED40 values for this antihyperalgesic and antiallodynic combined effect (13.83 +/- 0.65 mg/kg and 17.74 +/- 3.57 mg/kg; respectively) were significantly different. Coadministration of proglumide-Celecoxib showed an interaction index value of 0.45 +/- 0.03 for the antihyperalgesic effect and 0.39 +/- 0.08 for the antiallodynic activity, indicating a synergistic interaction. These data suggest that proglumide and Celecoxib can interact synergistically to reduce hyperalgesic and allodynic behaviors in diabetic neuropathy. This combination could be useful to treat neuropathic pain in diabetic patients. Drug Dev Res 78 : 116-123, 2017. (c)2017 Wiley Periodicals, Inc.
Chemoprevention of breast cancer in rats by celecoxib, a cyclooxygenase 2 inhibitor.[Pubmed:10786667]
Cancer Res. 2000 Apr 15;60(8):2101-3.
Nonsteroidal anti-inflammatory drugs (NSAIDs) have been observed to reduce the relative risk of breast cancer. This prompted our investigation of the chemopreventive potential of Celecoxib, a specific cyclooxygenase 2 blocker, against mammary carcinogenesis induced by 7,12-dimethyl-benz(a)anthracene in female Sprague Dawley rats. Treatment with Celecoxib was examined and compared to treatment with the general NSAID, ibuprofen, and to a control group receiving only dimethylbenz(a)anthracene. Dietary administration of Celecoxib (1500 ppm) produced striking reductions in the incidence, multiplicity, and volume of breast tumors relative to the control group (68%, 86%, and 81%, respectively; P < 0.001). Ibuprofen also produced significant effects, but of lesser magnitude (40%, 52%, and 57%, respectively; P < 0.001). These results help confirm the chemopreventive activity of NSAIDs against breast cancer and provide the first evidence that a cyclooxygenase 2 blocking agent, Celecoxib, possesses strong chemopreventive activity against mammary carcinogenesis.
Synthesis and biological evaluation of the 1,5-diarylpyrazole class of cyclooxygenase-2 inhibitors: identification of 4-[5-(4-methylphenyl)-3-(trifluoromethyl)-1H-pyrazol-1-yl]benze nesulfonamide (SC-58635, celecoxib).[Pubmed:9135032]
J Med Chem. 1997 Apr 25;40(9):1347-65.
A series of sulfonamide-containing 1,5-diarylpyrazole derivatives were prepared and evaluated for their ability to block cyclooxygenase-2 (COX-2) in vitro and in vivo. Extensive structure-activity relationship (SAR) work was carried out within this series, and a number of potent and selective inhibitors of COX-2 were identified. Since an early structural lead (1f, SC-236) exhibited an unacceptably long plasma half-life, a number of pyrazole analogs containing potential metabolic sites were evaluated further in vivo in an effort to identify compounds with acceptable pharmacokinetic profiles. This work led to the identification of 1i (4-[5-(4-methylphenyl)-3-(trifluoromethyl)- H-pyrazol-1-yl]benzenesulfonamide, SC-58635, Celecoxib), which is currently in phase III clinical trials for the treatment of rheumatoid arthritis and osteoarthritis.


