Ro 60-0175 fumaratePotent, selective 5-HT2C agonist CAS# 169675-09-6 |
- BS-181
Catalog No.:BCC1439
CAS No.:1092443-52-1
- WHI-P180 hydrochloride
Catalog No.:BCC4243
CAS No.:153437-55-9
- SNS-032 (BMS-387032)
Catalog No.:BCC1152
CAS No.:345627-80-7
- Dinaciclib (SCH727965)
Catalog No.:BCC3765
CAS No.:779353-01-4
- RGB-286638
Catalog No.:BCC5519
CAS No.:784210-87-3
- AT7519 Hydrochloride
Catalog No.:BCC1376
CAS No.:902135-91-5
Quality Control & MSDS
Number of papers citing our products

Chemical structure

3D structure
| Cas No. | 169675-09-6 | SDF | Download SDF |
| PubChem ID | 6446435 | Appearance | Powder |
| Formula | C15H16ClFN2O4 | M.Wt | 342.75 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 5 mM in water and to 20 mM in DMSO | ||
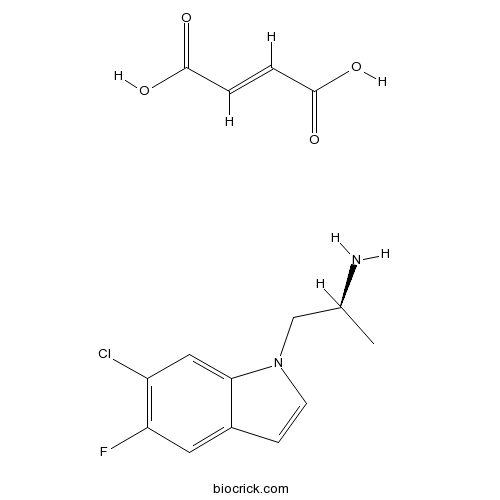
| Chemical Name | (E)-but-2-enedioic acid;(2S)-1-(6-chloro-5-fluoroindol-1-yl)propan-2-amine | ||
| SMILES | CC(CN1C=CC2=CC(=C(C=C21)Cl)F)N.C(=CC(=O)O)C(=O)O | ||
| Standard InChIKey | CEPHEXXZGQBXGT-XZTIXWOLSA-N | ||
| Standard InChI | InChI=1S/C11H12ClFN2.C4H4O4/c1-7(14)6-15-3-2-8-4-10(13)9(12)5-11(8)15;5-3(6)1-2-4(7)8/h2-5,7H,6,14H2,1H3;1-2H,(H,5,6)(H,7,8)/b;2-1+/t7-;/m0./s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Ro 60-0175 fumarate Dilution Calculator

Ro 60-0175 fumarate Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.9176 mL | 14.5879 mL | 29.1758 mL | 58.3516 mL | 72.9395 mL |
| 5 mM | 0.5835 mL | 2.9176 mL | 5.8352 mL | 11.6703 mL | 14.5879 mL |
| 10 mM | 0.2918 mL | 1.4588 mL | 2.9176 mL | 5.8352 mL | 7.2939 mL |
| 50 mM | 0.0584 mL | 0.2918 mL | 0.5835 mL | 1.167 mL | 1.4588 mL |
| 100 mM | 0.0292 mL | 0.1459 mL | 0.2918 mL | 0.5835 mL | 0.7294 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Z-Thr(tBu)-OH.DCHA
Catalog No.:BCC2565
CAS No.:16966-07-7
- Isodonal
Catalog No.:BCN3390
CAS No.:16964-56-0
- Trichosanatine
Catalog No.:BCN1818
CAS No.:169626-16-8
- Odoratone
Catalog No.:BCN1105
CAS No.:16962-90-6
- Celecoxib
Catalog No.:BCC1099
CAS No.:169590-42-5
- Deracoxib
Catalog No.:BCC4108
CAS No.:169590-41-4
- Floricaline
Catalog No.:BCN2104
CAS No.:16958-32-0
- Floridanine
Catalog No.:BCN2103
CAS No.:16958-31-9
- Florosenine
Catalog No.:BCN2108
CAS No.:16958-30-8
- Otosenine
Catalog No.:BCN2107
CAS No.:16958-29-5
- Fmoc-Ala(4-pyridyl)-OH
Catalog No.:BCC3327
CAS No.:169555-95-7
- IRL-2500
Catalog No.:BCC7192
CAS No.:169545-27-1
- Angelol K
Catalog No.:BCN8142
CAS No.:169736-93-0
- Dibutyryl-cAMP, sodium salt
Catalog No.:BCC8079
CAS No.:16980-89-5
- Mesuol
Catalog No.:BCN6583
CAS No.:16981-20-7
- Iso-cuparenal
Catalog No.:BCN7350
CAS No.:16982-01-7
- LY 333531 hydrochloride
Catalog No.:BCC7969
CAS No.:169939-93-9
- Reserpine hydrochloride
Catalog No.:BCC4279
CAS No.:16994-56-2
- Bufarenogin
Catalog No.:BCN2297
CAS No.:17008-65-0
- Pseudobufarenogin
Catalog No.:BCN8234
CAS No.:17008-69-4
- Alvimopan dihydrate
Catalog No.:BCC1348
CAS No.:170098-38-1
- Curcolone
Catalog No.:BCN3559
CAS No.:17015-43-9
- Nepicastat (SYN-117) HCl
Catalog No.:BCC2286
CAS No.:170151-24-3
- 11-Keto-beta-boswellic acid
Catalog No.:BCN2298
CAS No.:17019-92-0
Neuroendocrine evidence that (S)-2-(chloro-5-fluoro-indol- l-yl)-1-methylethylamine fumarate (Ro 60-0175) is not a selective 5-hydroxytryptamine(2C) receptor agonist.[Pubmed:12604698]
J Pharmacol Exp Ther. 2003 Mar;304(3):1209-16.
The 5-hydroxytryptamine(2A) and (2C) (5-HT(2A) and 5-HT(2C)) receptors are so closely related that selective agonists have not been developed until recently with the advent of (S)-2-(chloro-5-fluoro-indol-l-yl)-1-methylethylamine fumarate (Ro 60-0175), a putatively selective 5-HT(2C) receptor agonist. In the present study, Ro 60-0175 was used to analyze the importance of 5-HT(2C) receptors in hormone secretion. Injection of Ro 60-0175 (5 mg/kg s.c.) produced a maximum increase in plasma levels of adrenocorticotrophic hormone, oxytocin, and prolactin at 15 min postinjection and a maximum increase in plasma corticosterone levels at 60 min postinjection. Ro 60-0175-mediated increases in plasma hormone levels were dose-dependent (corticosterone ED(50) = 2.43 mg/kg; oxytocin ED(50) = 4.19 mg/kg; and prolactin ED(50) = 4.03 mg/kg). To assess the role of 5-HT(2C) and 5-HT(2A) receptors in mediating the hormone responses to Ro 60-0175, rats were pretreated with the 5-HT(2C) antagonist 6-chloro-5-methyl-1-[2-(2-methylpyridyl-3-oxy)-pyrid-5-yl carbonyl] indoline (SB 242084) or 5-HT(2A) antagonists (+/-)-2,3-dimethoxyphenyl-1-[2-4-(piperidine)-methanol] (MDL 100,907) before injection of Ro 60-0175 (5 mg/kg s.c.). Neither SB 242084 (0.1, 0.5, 1, and 5 mg/kg i.p.) nor MDL 100,907 (1, 5, and 10 microg/kg s.c.) significantly inhibited the Ro 60-0175-induced increases in plasma hormone levels. The data suggest that Ro 60-0175 increases hormone secretion by mechanisms independent of the activation of 5-HT(2C) and/or 5-HT(2A) receptors and suggest that Ro 60-0175 is not a highly selective 5-HT(2C) receptor agonist.
Design, synthesis, and pharmacological characterization of N- and O-substituted 5,6,7,8-tetrahydro-4H-isoxazolo[4,5-d]azepin-3-ol analogues: novel 5-HT(2A)/5-HT(2C) receptor agonists with pro-cognitive properties.[Pubmed:23301527]
J Med Chem. 2013 Feb 14;56(3):1211-27.
The isoxazol-3-one tautomer of the bicyclic isoxazole, 5,6,7,8-tetrahydro-4H-isoxazolo[4,5-d]azepin-3-ol (THAZ), has previously been shown to be a weak GABA(A) and glycine receptor antagonist. In the present study, the potential in this scaffold has been explored through the synthesis and pharmacological characterization of a series of N- and O-substituted THAZ analogues. The analogues N-Bn-THAZ (3d) and O-Bn-THAZ (4d) were found to be potent agonists of the human 5-HT(2A) and 5-HT(2C) receptors. Judging from an elaborate pharmacological profiling at numerous other CNS targets, the 3d analogue appears to be selective for the two receptors. Administration of 3d substantially improved the cognitive performance of mice in a place recognition Y-maze model, an effect fully reversible by coadministration of the selective 5-HT(2C) antagonist SB242084. In conclusion, as novel bioavailable cognitive enhancers that most likely mediate their effects through 5-HT(2A) and/or 5-HT(2C) receptors, the isoxazoles 3d and 4d constitute interesting leads for further medicinal chemistry development.
Effects of RO 60 0175, a 5-HT(2C) receptor agonist, in three animal models of anxiety.[Pubmed:10650160]
Eur J Pharmacol. 2000 Jan 10;387(2):197-204.
There is some controversy as to whether 5-HT(2C) receptor agonists are anxiogenic or anxiolytic. The effects of the novel 5-HT(2C) receptor agonist, (S)-2-chloro-5-fluoro-indol-1-yl)-1-methyl ethylamine fumarate (RO 60 0175), in three models of anxiety were therefore tested. RO 60 0175 was found to induce hypolocomotion in rats at doses greater than 0.5 mg/kg s.c., an effect reversed by the selective 5-HT(2C) receptor antagonist, SB-242084. RO 60 0175 did not elicit anxiolytic-like responses in the social interaction test under high light unfamiliar conditions, but suppressed both time spent in social interaction and locomotion at doses of 1 and 3 mg/kg s.c., suggesting a sedative response. In the Vogel conflict test, RO 60 0175 had no significant action on the number of shocks taken. In the Geller-Seifter test, RO 60 0175 (0.3 and 1 mg/kg s.c.) simultaneously reduced both unpunished and punished lever pressing, a profile consistent with sedation. Finally, RO 60 0175 was tested in a rat social interaction test under low light familiar conditions optimal for the detection of anxiogenic-like responses. At 1 and 3 mg/kg s.c., RO 60 0175 reduced both time spent in social interaction and concurrent locomotion, a profile more consistent with sedation than anxiogenesis. In conclusion, RO 60 0175 induced sedative-like responses via 5-HT(2C) receptor activation, but was neither anxiolytic, nor clearly anxiogenic at the doses tested.
5-HT2C receptor agonists: pharmacological characteristics and therapeutic potential.[Pubmed:9694950]
J Pharmacol Exp Ther. 1998 Aug;286(2):913-24.
In vitro, (S)-2-(chloro-5-fluoro-indol-1-yl)-1-methylethylamine 1:1 C4H4O4 and (S)-2-(4,4,7-trimethyl-1,4-dihydro-indeno[1, 2-b]pyrrol-1-yl)-1-methylethylamine 1:1 C4H4O4 exhibited high-affinity binding to the serotonin2C (5HT2C) receptors and stimulated turnover of inositol 1,4,5-triphosphate. Affinity to several of the other 5-HT receptor subtypes and to numerous nonserotonergic receptors was much lower. In rats, both compounds elicited behavioral signs of 5-HT2C receptor agonism but not 5-HT2A receptor agonism. Hypomotility induced in rats by high doses of these compounds was reversed by the 5-HT2C receptor antagonist N-(2-naphthyl)-N'-(3-pyridyl)-urea 1:1 HCI. In addition, these compounds were active in tests used to demonstrate anticompulsive effects: reducing schedule-induced polydipsia in rats (prevented by the 5-HT2C/2B receptor antagonist N-(1-methyl-5'-indolyl)-(3-pyridyl)urea 1:1 HCl, reversing increased scratching induced with 8-hydroxy-dipropylaminotetralin 1:1 HCl in squirrel monkeys (no tolerance developed), decreasing responding in the marble-burying task in mice, and decreasing excessive eating of palatable food in rats. In contrast to these compounds, fluoxetine was much less potent, and in some tasks less efficacious, in reducing excessive behavior in these models. These two 5-HT2C receptor agonists do not show anxiogenic effects in the plus-maze in rats. (S)-2-(4,4,7-trimethyl-1,4-dihydro-indeno[1, 2-b]pyrrol-1-yl)-1-methylethylamine 1:1 C4H4O4 reduced the olfactory bulbectomy-induced passive avoidance impairment in rats, a result that indicates antidepressant potential. Similarly, in the differential-reinforcement-of-low rate 72-s operant schedule task in rats, (S)-2-(chloro-5-fluoro-indol-1-yl)-1-methylethylamine 1:1 C4H4O4 increased (and (S)-2-(4,4,7-trimethyl-1,4-dihydro-indeno[1, 2-b]pyrrol-1-yl)-1-methylethylamine 1:1 C4H4O4 showed a tendency to increase) total reinforcements received, which is suggestive of antidepressant activity. The electroencephalography defined sleep-waking pattern in rats produced by these two 5-HT2C agonists, as well as fluoxetine, included increased quiet-waking and decreased rapid-eye-movement sleep, which is characteristic of antidepressant drugs. These results suggest that 5-HT2C receptor agonism is associated with therapeutic potential in obsessive compulsive disorder and depression.


