IsorhyncophyllineCAS# 6859-01-4 |
- Corynoxine B
Catalog No.:BCN8454
CAS No.:17391-18-3
- Isorhynchophylline
Catalog No.:BCN6458
CAS No.:6859-1-4
- Corynoxine
Catalog No.:BCN2364
CAS No.:6877-32-3
- Rhynchophylline
Catalog No.:BCN4979
CAS No.:76-66-4
Quality Control & MSDS
Number of papers citing our products

Chemical structure

3D structure
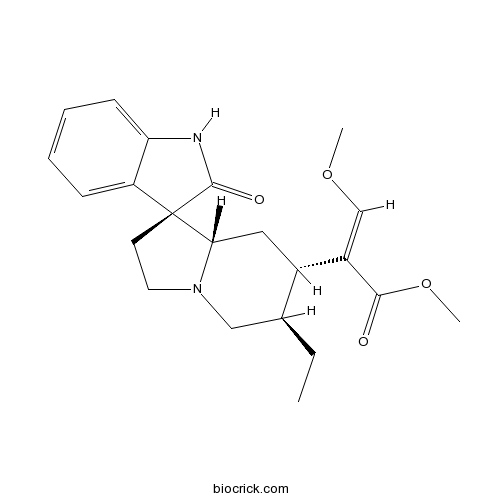
| Cas No. | 6859-01-4 | SDF | Download SDF |
| PubChem ID | 3037048 | Appearance | White powder |
| Formula | C22H28N2O4 | M.Wt | 384.5 |
| Type of Compound | Alkaloids | Storage | Desiccate at -20°C |
| Solubility | DMSO : 100 mg/mL (260.10 mM; Need ultrasonic) H2O : < 0.1 mg/mL (insoluble) | ||
| Chemical Name | methyl (E)-2-[(3S,6'R,7'S,8'aS)-6'-ethyl-2-oxospiro[1H-indole-3,1'-3,5,6,7,8,8a-hexahydro-2H-indolizine]-7'-yl]-3-methoxyprop-2-enoate | ||
| SMILES | CCC1CN2CCC3(C2CC1C(=COC)C(=O)OC)C4=CC=CC=C4NC3=O | ||
| Standard InChIKey | DAXYUDFNWXHGBE-VKCGGMIFSA-N | ||
| Standard InChI | InChI=1S/C22H28N2O4/c1-4-14-12-24-10-9-22(17-7-5-6-8-18(17)23-21(22)26)19(24)11-15(14)16(13-27-2)20(25)28-3/h5-8,13-15,19H,4,9-12H2,1-3H3,(H,23,26)/b16-13+/t14-,15-,19-,22-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Isorhyncophylline and rhyncophylline can directly inhibit the contractile responses induced by several agonists in small blood vessels of rat, they also can inhibit the hypertensive effect of angiotensin Ⅱ. |
| Targets | Potassium Channel |
| In vivo | Effects of rhyncophylline and isorhyncophylline on the contractile responses of rat isolated mesenteric arteries and tail artery[Reference: WebLink]Acta Academiae Medicinae Zunyi, 1994 (1) :7-10.
|
| Structure Identification | Phytochemistry.1984;23(2):453–455.Indole alkaloids from Hannoa klaineana roots.[Reference: WebLink]1-Methoxycanthin-6-one, rhyncophylline, Isorhyncophylline and four new alkaloids, ethyl-β-carboline-1-propionate, ethyl-β-carboline-2N-oxide-1-propionate, 1-ethyl-β-carboline and 1-ethyl-β-carboline-2N-oxide, were isolated from three different samples of Hannoa klaineana roots. |

Isorhyncophylline Dilution Calculator

Isorhyncophylline Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.6008 mL | 13.0039 mL | 26.0078 mL | 52.0156 mL | 65.0195 mL |
| 5 mM | 0.5202 mL | 2.6008 mL | 5.2016 mL | 10.4031 mL | 13.0039 mL |
| 10 mM | 0.2601 mL | 1.3004 mL | 2.6008 mL | 5.2016 mL | 6.502 mL |
| 50 mM | 0.052 mL | 0.2601 mL | 0.5202 mL | 1.0403 mL | 1.3004 mL |
| 100 mM | 0.026 mL | 0.13 mL | 0.2601 mL | 0.5202 mL | 0.6502 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Isorhynchophylline (IRN), an alkaloid isolated from Uncaria rhynchophylla, possesses the effects of lowered blood pressure, vasodilatation and protection against ischemia-induced neuronal damage. IC50 value: Target: In vitro: Isorhynchophylline concentration-dependently inhibited the platelet-derived growth factor (PDGF)-BB-induced proliferation of PASMCs. Fluorescence-activated cell-sorting analysis showed that isorhynchophylline caused G0/G1 phase cell cycle arrest [2]. Isorhynchophylline can significantly attenuate the cardiomyocyte hypertrophy induced by AngⅡ [3]. In vivo: Isorhynchophylline significantly improved spatial learning and memory function in the D-gal-treated mice. Isorhynchophylline significantly increased the level of glutathione (GSH) and the activities of superoxide dismutase (SOD) and catalase (CAT), while decreased the level of malondialdehyde (MDA) in the brain tissues of the D-gal-treated mice [1]. Isorhynchophylline prevented monocrotaline induced pulmonary arterial hypertension in rats, as assessed by right ventricular (RV) pressure, the weight ratio of RV to (left ventricular+septum) and RV hypertrophy. Isorhynchophylline significantly attenuated the percentage of fully muscularized small arterioles, the medial wall thickness, and the expression of smooth muscle α-actin (α-SMA) and proliferating cell nuclear antigen (PCNA) [2].
References:
[1]. Yan-Fang Xian, et al. Isorhynchophylline improves learning and memory impairments induced by D-galactose in mice. Neurochemistry International Volume 76, October 2014, Pages 42–49
[2]. Guo H, et al. Isorhynchophylline protects against pulmonary arterial hypertension and suppresses PASMCs proliferation. Biochem Biophys Res Commun. 2014 Jul 18;450(1):729-34.
[3]. LI Qiang, et al. Inhibitory effect of isorhynchophylline on cardiomyocyte hypertrophy induced by angiotensinⅡ. West China JOurnal of Pharmaceutical Sciences, 2013-05
- PX-478 2HCl
Catalog No.:BCC6502
CAS No.:685898-44-6
- Prometaphanine
Catalog No.:BCN4244
CAS No.:6858-85-1
- Moschamine
Catalog No.:BCN3900
CAS No.:68573-23-9
- Pridinol Methanesulfonate
Catalog No.:BCC3845
CAS No.:6856-31-1
- Eupatoriopicrin
Catalog No.:BCN7116
CAS No.:6856-01-5
- GSK 264220A
Catalog No.:BCC6062
CAS No.:685506-42-7
- Cilostamide
Catalog No.:BCC6843
CAS No.:68550-75-4
- Isoguvacine hydrochloride
Catalog No.:BCC6575
CAS No.:68547-97-7
- Angiotensin II
Catalog No.:BCC1030
CAS No.:68521-88-0
- Vigabatrin
Catalog No.:BCC2039
CAS No.:68506-86-5
- Pramiracetam
Catalog No.:BCC4928
CAS No.:68497-62-1
- WS 12
Catalog No.:BCC7543
CAS No.:68489-09-8
- Isorhynchophylline
Catalog No.:BCN6458
CAS No.:6859-1-4
- Xylobiose
Catalog No.:BCN8424
CAS No.:6860-47-5
- Procerine
Catalog No.:BCN2017
CAS No.:68622-81-1
- Otenabant
Catalog No.:BCC1828
CAS No.:686344-29-6
- CP-945598 HCl
Catalog No.:BCC1082
CAS No.:686347-12-6
- BOP-Cl
Catalog No.:BCC2808
CAS No.:68641-49-6
- (±)-Palmitoylcarnitine chloride
Catalog No.:BCC6718
CAS No.:6865-14-1
- IWP-2
Catalog No.:BCC1665
CAS No.:686770-61-6
- BC 11-38
Catalog No.:BCC7940
CAS No.:686770-80-9
- IWP 4
Catalog No.:BCC5602
CAS No.:686772-17-8
- Qianhucoumarin G
Catalog No.:BCN3704
CAS No.:68692-61-5
- Retronecine N-oxide
Catalog No.:BCN2035
CAS No.:6870-33-3
Aqueous extracts from Uncaria tomentosa (Willd. ex Schult.) DC. reduce bronchial hyperresponsiveness and inflammation in a murine model of asthma.[Pubmed:29432856]
J Ethnopharmacol. 2018 May 23;218:76-89.
ETHNOPHARMACOLOGICAL RELEVANCE: Uncaria tomentosa (Willd. Ex Schult) DC is used by indigenous tribes in the Amazonian region of Central and South America to treat inflammation, allergies and asthma. The therapeutic properties of U. tomentosa have been attributed to the presence of tetracyclic and pentacyclic oxindole alkaloids and to phenolic acids. AIMS OF THE STUDY: To characterize aqueous bark extracts (ABE) and aqueous leaf extracts (ALE) of U. tomentosa and to compare their anti-inflammatory effects. MATERIALS AND METHODS: Constituents of the extracts were identified by ultra performance liquid chromatography-mass spectrometry. Anti-inflammatory activities were assessed in vitro by exposing lipopolysaccharide-stimulated macrophage cells (RAW264.7-Luc) to ABE, ALE and standard mitraphylline. In vivo assays were performed using a murine model of ovalbumin (OVA)-induced asthma. OVA-sensitized animals were treated with ABE or ALE while controls received dexamethasone or saline solution. Bronchial hyperresponsiveness, production of Th1 and Th2 cytokines, total and differential counts of inflammatory cells in the bronchoalveolar lavage (BAL) and lung tissue were determined. RESULTS: Mitraphylline, isomitraphylline, chlorogenic acid and quinic acid were detected in both extracts, while Isorhyncophylline and rutin were detected only in ALE. ABE, ALE and mitraphylline inhibited the transcription of nuclear factor kappa-B in cell cultures, ALE and mitraphylline reduced the production of interleukin (IL)-6, and mitraphylline reduced production of tumor necrosis factor-alpha. Treatment with ABE and ALE at 50 and 200mgkg(-1), respectively, reduced respiratory elastance and tissue damping and elastance. ABE and ALE reduced the number of eosinophils in BAL, while ALE at 200mgkg(-1) reduced the levels of IL-4 and IL-5 in the lung homogenate. Peribronchial inflammation was significantly reduced by treatment with ABE and ALE at 50 and 100mgkg(-1) respectively. CONCLUSION: The results clarify for the first time the anti-inflammatory activity of U. tomentosa in a murine model of asthma. Although ABE and ALE exhibited distinct chemical compositions, both extracts inhibited the production of pro-inflammatory cytokines in vitro. In vivo assays revealed that ABE was more effective in treating asthmatic inflammation while ALE was more successful in controlling respiratory mechanics. Both extracts may have promising applications in the phytotherapy of allergic asthma.


