JNJ 16259685Extremely potent, mGlu1-selective non-competitive antagonist CAS# 409345-29-5 |
- Cyclopamine
Catalog No.:BCN2964
CAS No.:4449-51-8
- Purmorphamine
Catalog No.:BCC3641
CAS No.:483367-10-8
- GANT61
Catalog No.:BCC1090
CAS No.:500579-04-4
- GANT 58
Catalog No.:BCC1587
CAS No.:64048-12-0
- GDC-0449 (Vismodegib)
Catalog No.:BCC1285
CAS No.:879085-55-9
Quality Control & MSDS
Number of papers citing our products

Chemical structure

3D structure
| Cas No. | 409345-29-5 | SDF | Download SDF |
| PubChem ID | 11313361 | Appearance | Powder |
| Formula | C20H23NO3 | M.Wt | 325.41 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : ≥ 100 mg/mL (307.31 mM) *"≥" means soluble, but saturation unknown. | ||
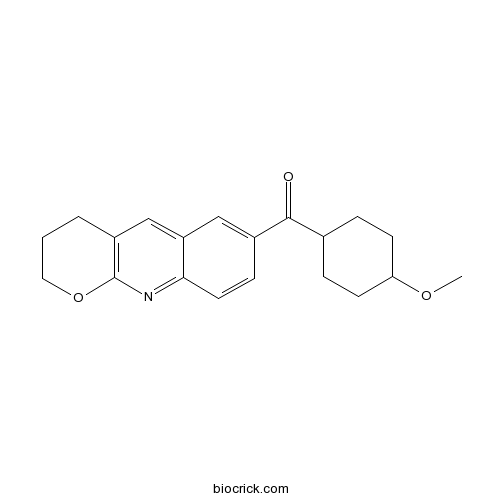
| Chemical Name | 3,4-dihydro-2H-pyrano[2,3-b]quinolin-7-yl-(4-methoxycyclohexyl)methanone | ||
| SMILES | COC1CCC(CC1)C(=O)C2=CC3=CC4=C(N=C3C=C2)OCCC4 | ||
| Standard InChIKey | QOTAQTRFJWLFCR-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C20H23NO3/c1-23-17-7-4-13(5-8-17)19(22)14-6-9-18-16(11-14)12-15-3-2-10-24-20(15)21-18/h6,9,11-13,17H,2-5,7-8,10H2,1H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Sub-nanomolar potent, non-competitive mGlu1 antagonist (Ki = 0.34 nM). Inhibits glutamate-induced Ca2+ response at the human mGlu1 receptor with an IC50 value of 0.55 nM. Selective over mGlu5 (> 400-fold) and displays no activity at mGlu2, mGlu3, mGlu4, mGlu6, AMPA or NMDA receptors (IC50 > 10 μM). Centrally active following systemic administration. |

JNJ 16259685 Dilution Calculator

JNJ 16259685 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.073 mL | 15.3652 mL | 30.7305 mL | 61.4609 mL | 76.8262 mL |
| 5 mM | 0.6146 mL | 3.073 mL | 6.1461 mL | 12.2922 mL | 15.3652 mL |
| 10 mM | 0.3073 mL | 1.5365 mL | 3.073 mL | 6.1461 mL | 7.6826 mL |
| 50 mM | 0.0615 mL | 0.3073 mL | 0.6146 mL | 1.2292 mL | 1.5365 mL |
| 100 mM | 0.0307 mL | 0.1537 mL | 0.3073 mL | 0.6146 mL | 0.7683 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
JNJ16259685 is a selective antagonist of mGlu1 receptor, and inhibits the synaptic activation of mGlu1 in a concentration-dependent manner with IC50 of 19 nM.
In Vitro:JNJ16259685 potently and completely inhibits the glutamate (30 μM)-induced increase in intracellular Ca2+ concentrations at the rat mGlu1a receptor with an IC50 value of 3.24±1.00 nM. IC50 values for CPCCOEt and BAY 36-7620 are 17.8±10.3 μM and 161±38 nM, respectively. The potency of JNJ16259685 in blocking glutamate (30 μM)-induced Ca2+ mobilization at the human mGlu1a receptor is 1.21±0.53 nM (IC50 n=3). JNJ16259685 inhibits the glutamate (3 μM)-induced rise in intracellular Ca2+ concentrations at the rat mGlu5a receptor with an IC50 value of 1.31±0.39 μM (n=4). JNJ16259685 blocks glutamate (3 μM)-induced Ca2+ mobilization at the human mGlu5 receptor with an IC50 of 28.3±11.7 μM (n=4). JNJ16259685 does not exhibit agonist activity at any of the group I mGlu receptors[3].
In Vivo:JNJ16259685 (0.125, 0.25, 0.5, 1, 2, 4 and 8 mg/kg, i.p) significantly reduces the time spent in digging behaviours (0.25-8 mg/kg), threat (all doses) and attack, in comparison with vehicle group[1]. JNJ16259685 (30 mg/kg) produces very minimal effects on locomotor activity. JNJ16259685 dramatically reduces rearing behavior, exploration of a novel environment and lever pressing for a food reward (rat: 0.3 mg/kg; mouse: 1 mg/kg). Subcutaneously administered JNJ16259685 (30 mg/kg) has no effect on reflexive startle responses to loud auditory stimuli or foot shock in mice[2]. JNJ16259685 exhibits high potencies in occupying central mGlu1 receptors in the rat cerebellum and thalamus (ED50=0.040 and 0.014 mg/kg, respectively)[3].
References:
[1]. Navarro JF,et al. JNJ16259685, a selective mGlu1 antagonist, suppresses isolation-induced aggression in male mice. Eur J Pharmacol. 2008 May 31;586(1-3):217-20.
[2]. Hodgson RA, et al. Characterization of the selective mGluR1 antagonist, JNJ16259685, in rodent models of movement and coordination. Pharmacol Biochem Behav. 2011 Apr;98(2):181-7.
[3]. Lavreysen H,et al. JNJ16259685, a highly potent, selective and systemically active mGlu1 receptor antagonist. Neuropharmacology. 2004 Dec;47(7):961-72.
- 3,4-Dimethoxycinnamyl alcohol
Catalog No.:BCN6499
CAS No.:40918-90-9
- Sinoacutine
Catalog No.:BCN5461
CAS No.:4090-18-0
- H-Tyr-OEt.HCl
Catalog No.:BCC3125
CAS No.:4089-07-0
- 3,5-Dihydroxy-1,7-bis(3,4-dihydroxyphenyl)heptane
Catalog No.:BCN7494
CAS No.:408324-01-6
- 3,5-Dihydroxy-1-(3,4-dihydroxyphenyl)-7-(4-hydroxyphenyl)heptane
Catalog No.:BCN7165
CAS No.:408324-00-5
- Suprofen
Catalog No.:BCC4943
CAS No.:40828-46-4
- MDL 72222
Catalog No.:BCC6733
CAS No.:40796-97-2
- H-D-Asp(OBzl)-OBzl.TosOH
Catalog No.:BCC2900
CAS No.:4079-64-5
- PD 146176
Catalog No.:BCC7504
CAS No.:4079-26-9
- FPL 55712
Catalog No.:BCC7310
CAS No.:40785-97-5
- 1,7-Diepi-8,15-cedranediol
Catalog No.:BCN5460
CAS No.:40768-81-8
- CaCCinh-A01
Catalog No.:BCC6314
CAS No.:407587-33-1
- VU0152100
Catalog No.:BCC4053
CAS No.:409351-28-6
- Taxiresinol
Catalog No.:BCN4637
CAS No.:40951-69-7
- Glycitein
Catalog No.:BCN5896
CAS No.:40957-83-3
- Medioresinol
Catalog No.:BCN5462
CAS No.:40957-99-1
- Desacetylcinobufotalin
Catalog No.:BCC8166
CAS No.:4099-30-3
- H-D-Ala-OBzl.TosOH
Catalog No.:BCC2850
CAS No.:41036-32-2
- Palovarotene
Catalog No.:BCC4185
CAS No.:410528-02-8
- Sirtinol
Catalog No.:BCC2224
CAS No.:410536-97-9
- Timosaponin A3
Catalog No.:BCN4999
CAS No.:41059-79-4
- Lipiferolide
Catalog No.:BCN5463
CAS No.:41059-80-7
- Neobavaisoflavone
Catalog No.:BCN3194
CAS No.:41060-15-5
- Skullcapflavone I
Catalog No.:BCN5464
CAS No.:41060-16-6
The novel anticancer agent JNJ-26854165 is active in chronic myeloid leukemic cells with unmutated BCR/ABL and T315I mutant BCR/ABL through promoting proteosomal degradation of BCR/ABL proteins.[Pubmed:27999193]
Oncotarget. 2017 Jan 31;8(5):7777-7790.
Chronic myeloid leukemia (CML) is a clonal malignant disease caused by the expression of BCR/ABL. MDM2 (human homolog of the murine double minute-2) inhibitors such as Nutlin-3 have been shown to induce apoptosis in a p53-dependent manner in CML cells and sensitize cells to Imatinib. Here, we demonstrate that JNJ-26854165, an inhibitor of MDM2, inhibits proliferation and triggers cell death in a p53-independent manner in various BCR/ABL-expressing cells, which include primary leukemic cells from patients with CML blast crisis and cells expressing the Imatinib-resistant T315I BCR/ABL mutant. The response to JNJ-26854165 is associated with the downregulation of BCR/ABL dependently of proteosome activation. Moreover, in all tested CML cells, with the exception of T315I mutation cells, combining JNJ-26854165 and tyrosine kinase inhibitor (TKI) Imatinib or PD180970 leads to a synergistic effect. In conclusion, our results suggest that JNJ-26854165, used either alone or in combination with TKIs, represents a promising novel targeted approach to overcome TKI resistance and improve patient outcome in CML.
Population Pharmacokinetic Modeling of JNJ-53718678, a Novel Fusion Inhibitor for the Treatment of Respiratory Syncytial Virus: Results from a Phase I, Double-Blind, Randomized, Placebo-Controlled First-in-Human Study in Healthy Adult Subjects.[Pubmed:28238203]
Clin Pharmacokinet. 2017 Nov;56(11):1331-1342.
BACKGROUND: JNJ-53718678 is a potent small-molecule inhibitor of the F-glycoprotein-mediated complex membrane fusion process of the respiratory syncytial virus. Here, we report the pharmacokinetics, the population pharmacokinetic modeling, and the safety and tolerability of JNJ-53718678 from the first-in-human, double-blind, randomized, placebo-controlled phase I study. METHODS: Healthy subjects were randomized (6:3) into five single-dose groups (25-1000 mg) or three multiple-dose groups [250 mg every 24 h (q24h), 500 mg q24h, 250 mg every 12 h; fed conditions for 8 days] to receive JNJ-53718678 or placebo. Blood and urine samples were collected at several timepoints up to 72 h after intake of JNJ-53718678 and analyzed using validated liquid chromatography-mass spectrometry methods. A population pharmacokinetic model was developed and validated. RESULTS: Peak plasma concentrations of JNJ-53718678 increased with increasing single (159 +/- 54.9 to 6702 +/- 1733 ng/mL) and multiple (on day 8, 1528 +/- 256 to 2655 +/- 591 ng/mL) doses. Steady-state conditions were reached on day 2 of the 8-day dosing regimen. Less than 4% of JNJ-53718678 was excreted in urine across all dose groups. Mean exposure of JNJ-53718678 was 7% lower in the fed state compared with the fasted state at the same dose. A two-compartment model with first-order absorption with parallel linear and non-linear elimination best described the pharmacokinetics of JNJ-53718678. No covariate effects were observed. CONCLUSIONS: A population pharmacokinetic model that describes the concentration data well with good precision of all parameter estimates was developed and validated. JNJ-53718678 was well tolerated at all single and multiple doses studied.
Efficacy, safety and pharmacokinetics of simeprevir and TMC647055/ritonavir with or without ribavirin and JNJ-56914845 in HCV genotype 1 infection.[Pubmed:28187751]
BMC Gastroenterol. 2017 Feb 10;17(1):26.
BACKGROUND: A Phase 2a, open-label study (NCT01724086) was conducted to assess the efficacy and safety of a once-daily, 2-direct-acting-antiviral-agent (2-DAA) combination of simeprevir + TMC647055/ritonavir +/- ribavirin and of the 3-DAA combination of simeprevir + TMC647055/ritonavir + JNJ-56914845 in chronic hepatitis C virus genotype (GT)1-infected treatment-naive and prior-relapse patients. METHODS: The study comprised four 12-week treatment panels: Panel 1 (n = 10; GT1a) and Panel 2-Arm 1 (n = 12; GT1b): simeprevir 75 mg once daily + TMC647055 450 mg once daily/ritonavir 30 mg once daily + ribavirin 1000-1200 mg/day; Panel 2-Arm 2 (n = 9; GT1b): simeprevir 75 mg + TMC647055 450 mg/ritonavir 30 mg without ribavirin; Panel 3: simeprevir 75 mg + TMC647055 600 mg/ritonavir 50 mg with (Arm 1: GT1a; n = 7) or without (Arm 2: GT1b; n = 8) ribavirin; Panel 4: simeprevir 75 mg + TMC647055 450 mg/ritonavir 30 mg + JNJ-56914845 30 mg once daily (Arm 1: n = 22; GT1a/GT1b) or 60 mg once daily (Arm 2: n = 22; GT1a/GT1b). Primary endpoint was sustained virologic response 12 weeks after end of treatment (12 weeks of combination treatment; SVR12). RESULTS: In Panel 1 and Panel 2-Arm 1, 5/10 and 6/12 (50%) GT1a/GT1b + ribavirin patients achieved SVR12, versus 3/9 (33%) GT1b without ribavirin patients in Panel 2-Arm 2. In Panel 3-Arm 1 and Panel 3-Arm 2, 6/7 (86%) GT1a + ribavirin and 4/8 (50%) GT1b without ribavirin patients, respectively, achieved SVR12. In Panel 4, 10/14 (71%) and 14/15 (93%) GT1a patients in Arms 1 and 2 achieved SVR12 compared with 8/8 and 7/7 (100%) GT1b patients in each arm, respectively. No deaths, serious adverse events (AEs), Grade 4 AEs or AEs leading to treatment discontinuation occurred. CONCLUSIONS: The 2- and 3-DAA combinations were well tolerated. High SVR rates of 93% and 100% in GT1a- and GT1b-infected patients, respectively, were achieved in this study by combining simeprevir with JNJ-56914845 60 mg and TMC647055/ritonavir. TRIAL REGISTRATION: NCT01724086 (date of registration: September 26, 2012).
Discovery and Pharmacological Characterization of JNJ-42756493 (Erdafitinib), a Functionally Selective Small-Molecule FGFR Family Inhibitor.[Pubmed:28341788]
Mol Cancer Ther. 2017 Jun;16(6):1010-1020.
Fibroblast growth factor (FGF) signaling plays critical roles in key biological processes ranging from embryogenesis to wound healing and has strong links to several hallmarks of cancer. Genetic alterations in FGF receptor (FGFR) family members are associated with increased tumor growth, metastasis, angiogenesis, and decreased survival. JNJ-42756493, erdafitinib, is an orally active small molecule with potent tyrosine kinase inhibitory activity against all four FGFR family members and selectivity versus other highly related kinases. JNJ-42756493 shows rapid uptake into the lysosomal compartment of cells in culture, which is associated with prolonged inhibition of FGFR signaling, possibly due to sustained release of the inhibitor. In xenografts from human tumor cell lines or patient-derived tumor tissue with activating FGFR alterations, JNJ-42756493 administration results in potent and dose-dependent antitumor activity accompanied by pharmacodynamic modulation of phospho-FGFR and phospho-ERK in tumors. The results of the current study provide a strong rationale for the clinical investigation of JNJ-42756493 in patients with tumors harboring FGFR pathway alterations. Mol Cancer Ther; 16(6); 1010-20. (c)2017 AACR.
Effects of mGluR1 antagonism in the dorsal hippocampus on drug context-induced reinstatement of cocaine-seeking behavior in rats.[Pubmed:19847405]
Psychopharmacology (Berl). 2010 Jan;208(1):1-11.
RATIONALE: The functional integrity of the dorsal hippocampus (DH) is necessary for drug context-induced reinstatement of cocaine seeking. However, the neuropharmacological mechanisms of this phenomenon are poorly understood. OBJECTIVES: Given the known significance of group I metabotropic glutamate receptors (mGluRs), including the mGluR1 subtype, in drug-induced behaviors, the present study was designed to evaluate the contribution of mGluR1s in the DH to drug context-induced reinstatement of extinguished cocaine-seeking behavior. METHODS: Sprague-Dawley rats were trained to lever press for unsignaled cocaine infusions in a distinct environmental context (cocaine-paired context) followed by extinction training in a distinctly different environmental context (extinction context). Using a counterbalanced partial within-subjects testing design, rats were re-exposed to the cocaine-paired context or the extinction context while cocaine-seeking behavior (nonreinforced active lever pressing) was assessed. Prior to each test session, rats received bilateral microinfusions of the highly potent mGluR1-selective antagonist JNJ16259685 (0.6, 30, or 120 pg/0.5 microl per hemisphere) or vehicle into the DH or the overlying somatosensory cortex trunk region (SStr; anatomical control). RESULTS: Intra-DH, but not intra-SStr, JNJ16259685 infusions dose dependently attenuated drug context-induced reinstatement of cocaine seeking relative to vehicle treatment, without attenuating instrumental behavior in the extinction context, general motor activity, or food-reinforced instrumental behavior in control experiments. CONCLUSIONS: Stimulation of mGluR1s in the DH is necessary for incentive motivational and/or memory processes that contribute to drug context-induced cocaine-seeking behavior. These findings indicate that the mGluR1 is an interesting target from an addiction treatment perspective.
Synthesis, structure-activity relationship, and receptor pharmacology of a new series of quinoline derivatives acting as selective, noncompetitive mGlu1 antagonists.[Pubmed:15771457]
J Med Chem. 2005 Mar 24;48(6):2134-53.
We describe the discovery and the structure-activity relationship of a new series of quinoline derivatives acting as selective and highly potent noncompetitive mGlu1 antagonists. We first identified cis-10 as a fairly potent mGlu1 antagonist (IC(50) = 20 nM) in a cell-based signal transduction assay on the rat mGlu1 receptor expressed in CHO-K1 cells, and then we were able to design and synthesize highly potent compounds on both rat and human mGlu1 receptors as exemplified by compound cis-64a, which has an antagonist potency of 0.5 nM for the human mGlu1 receptor. We briefly present and discuss the in vitro metabolic stability of the compounds in human liver microsomes. We finally report the pharmacokinetic properties of our lead compound cis-64a.
Metabotropic glutamate receptor 1 blockade impairs acquisition and retention in a spatial Water maze task.[Pubmed:16043241]
Behav Brain Res. 2005 Oct 14;164(1):52-60.
Metabotropic glutamate receptors, including the mGlu1 receptor, have received considerable attention as potential targets for anxiolytic, antidepressant, antipsychotic and antinociceptive drugs. mGlu1 receptors have also been suggested to play a role in the modulation of cognitive processes, but knowledge is still very limited. In the present study the effects of the selective mGlu1 receptor antagonist 3,4-dihydro-2H-pyrano[2,3]beta-quinolin-7-yl)(cis-4-methoxycyclohexyl)methanone (JNJ16259685, 0.63-10 mg/kg s.c.) on more or less spatially demanding learning and spatial memory (retention and re-acquisition) were investigated in mice performing in a water maze. Selective mGlu1 receptor blockade with JNJ16259685 impaired spatial acquisition processes, irrespective of spatial load, as well as spatial re-acquisition, already at the lowest dose tested (0.63 mg/kg). In contrast, effects on spatial retention performance were relatively mild in mice that had learned to locate the position of the escape platform prior to treatment. Thigmotaxic behaviour and locomotor activity appeared to be unaffected by JNJ16259685. These data suggest that blockade of the mGlu1 receptor primarily affects learning of new information, but leaves retention of spatial information relatively unaffected. Blockade of the mGlu5 receptor with MPEP also impaired spatial learning, although only at the highest dose tested (10 mg/kg). An ex vivo receptor occupancy study in rats revealed that MPEP occupied central mGlu5 receptors with an ED(50) of 2.0 mg/kg one hour after subcutaneous administration. This is 50-150 times higher than the ED(50) reported for JNJ16259685 at central mGlu1 receptors and suggests that one reason why the two compounds cause cognitive effects at different doses might be due to differences in central mGlu receptor occupancy, rather than fundamentally different roles of mGlu1 and mGlu5 receptors in the modulation of cognitive function.
JNJ16259685, a highly potent, selective and systemically active mGlu1 receptor antagonist.[Pubmed:15555631]
Neuropharmacology. 2004 Dec;47(7):961-72.
We examined the pharmacological profile of (3,4-dihydro-2H-pyrano[2,3]b quinolin-7-yl) (cis-4-methoxycyclohexyl) methanone (JNJ16259685). At recombinant rat and human metabotropic glutamate (mGlu) 1a receptors, JNJ16259685 non-competitively inhibited glutamate-induced Ca2+ mobilization with IC50 values of 3.24+/-1.00 and 1.21+/-0.53 nM, respectively, while showing a much lower potency at the rat and human mGlu5a receptor. JNJ16259685 inhibited [3H]1-(3,4-dihydro-2H-pyrano[2,3-b]quinolin-7-yl)-2-phenyl-1-ethanone ([3H]R214127) binding to membranes prepared from cells expressing rat mGlu1a receptors with a Ki of 0.34+/-0.20 nM. JNJ16259685 showed no agonist, antagonist or positive allosteric activity toward rat mGlu2, -3, -4 or -6 receptors at concentrations up to 10 microM and did not bind to AMPA or NMDA receptors, or to a battery of other neurotransmitter receptors, ion channels and transporters. In primary cerebellar cultures, JNJ16259685 inhibited glutamate-mediated inositol phosphate production with an IC50 of 1.73+/-0.40 nM. Subcutaneously administered JNJ16259685 exhibited high potencies in occupying central mGlu1 receptors in the rat cerebellum and thalamus ( ED50=0.040 and 0.014 mg/kg, respectively). These data show that JNJ16259685 is a selective mGlu1 receptor antagonist with excellent potencies in inhibiting mGlu1 receptor function and binding and in occupying the mGlu1 receptor after systemic administration.


