PD 146176Selective 15-lipoxygenase inhibitor CAS# 4079-26-9 |
- Irbesartan
Catalog No.:BCC2560
CAS No.:138402-11-6
- Olmesartan
Catalog No.:BCC1819
CAS No.:144689-24-7
- AVE 0991
Catalog No.:BCC4032
CAS No.:304462-19-9
- Tranilast
Catalog No.:BCC2514
CAS No.:53902-12-8
Quality Control & MSDS
Number of papers citing our products

Chemical structure

3D structure
| Cas No. | 4079-26-9 | SDF | Download SDF |
| PubChem ID | 297589 | Appearance | Powder |
| Formula | C15H11NS | M.Wt | 237.32 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : 100 mg/mL (421.37 mM; Need ultrasonic) | ||
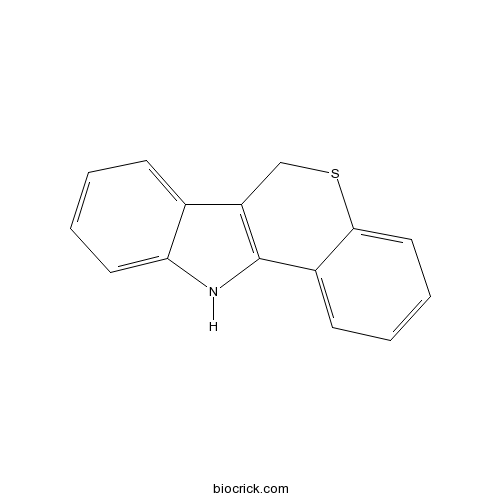
| Chemical Name | 6,11-dihydrothiochromeno[4,3-b]indole | ||
| SMILES | C1C2=C(C3=CC=CC=C3S1)NC4=CC=CC=C24 | ||
| Standard InChIKey | ZGOOPZVQMLHPFM-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C15H11NS/c1-3-7-13-10(5-1)12-9-17-14-8-4-2-6-11(14)15(12)16-13/h1-8,16H,9H2 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Specific, non-competitve 15-lipoxygenase (15-LOX) inhibitor (Ki = 197nM) that has no demonstrable effect on 5-LOX, 12-LOX, COX-1 or COX-2 (IC50 = 0.54 μM for 15-LOX in rabbit reticulocytes). Lacks non-specific antioxidant properties and prevents atherogenesis via regulation of monocyte-macrophage enrichment in vivo. |

PD 146176 Dilution Calculator

PD 146176 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 4.2137 mL | 21.0686 mL | 42.1372 mL | 84.2744 mL | 105.343 mL |
| 5 mM | 0.8427 mL | 4.2137 mL | 8.4274 mL | 16.8549 mL | 21.0686 mL |
| 10 mM | 0.4214 mL | 2.1069 mL | 4.2137 mL | 8.4274 mL | 10.5343 mL |
| 50 mM | 0.0843 mL | 0.4214 mL | 0.8427 mL | 1.6855 mL | 2.1069 mL |
| 100 mM | 0.0421 mL | 0.2107 mL | 0.4214 mL | 0.8427 mL | 1.0534 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- FPL 55712
Catalog No.:BCC7310
CAS No.:40785-97-5
- 1,7-Diepi-8,15-cedranediol
Catalog No.:BCN5460
CAS No.:40768-81-8
- CaCCinh-A01
Catalog No.:BCC6314
CAS No.:407587-33-1
- (+)-Nerolidol
Catalog No.:BCC8219
CAS No.:142-50-7
- (-)-Bicuculline methiodide
Catalog No.:BCC7387
CAS No.:40709-69-1
- Actinine
Catalog No.:BCN1744
CAS No.:407-64-7
- O-Phospho-L-serine
Catalog No.:BCC6578
CAS No.:407-41-0
- Taxifolin 3-O-beta-D-xylopyranoside
Catalog No.:BCN5458
CAS No.:40672-47-7
- Cornoside
Catalog No.:BCN7575
CAS No.:40661-45-8
- IKK-2 inhibitor VIII
Catalog No.:BCC1642
CAS No.:406209-26-5
- ACHP
Catalog No.:BCC6223
CAS No.:406208-42-2
- DSP-4
Catalog No.:BCC7527
CAS No.:40616-75-9
- H-D-Asp(OBzl)-OBzl.TosOH
Catalog No.:BCC2900
CAS No.:4079-64-5
- MDL 72222
Catalog No.:BCC6733
CAS No.:40796-97-2
- Suprofen
Catalog No.:BCC4943
CAS No.:40828-46-4
- 3,5-Dihydroxy-1-(3,4-dihydroxyphenyl)-7-(4-hydroxyphenyl)heptane
Catalog No.:BCN7165
CAS No.:408324-00-5
- 3,5-Dihydroxy-1,7-bis(3,4-dihydroxyphenyl)heptane
Catalog No.:BCN7494
CAS No.:408324-01-6
- H-Tyr-OEt.HCl
Catalog No.:BCC3125
CAS No.:4089-07-0
- Sinoacutine
Catalog No.:BCN5461
CAS No.:4090-18-0
- 3,4-Dimethoxycinnamyl alcohol
Catalog No.:BCN6499
CAS No.:40918-90-9
- JNJ 16259685
Catalog No.:BCC7332
CAS No.:409345-29-5
- VU0152100
Catalog No.:BCC4053
CAS No.:409351-28-6
- Taxiresinol
Catalog No.:BCN4637
CAS No.:40951-69-7
- Glycitein
Catalog No.:BCN5896
CAS No.:40957-83-3
Co-expression of AFAP1-AS1 and PD-1 predicts poor prognosis in nasopharyngeal carcinoma.[Pubmed:28380458]
Oncotarget. 2017 Jun 13;8(24):39001-39011.
Nasopharyngeal carcinoma (NPC) carries a high potential for metastasis and immune escape, with a great risk of relapse after primary treatment. Through analysis of whole genome expression profiling data in NPC samples, we found that the expression of a long non-coding RNA (lncRNA), actin filament-associated protein 1 antisense RNA 1 (AFAP1-AS1), is significantly correlated with the immune escape marker programmed death 1 (PD-1). We therefore assessed the expression of AFAP1-AS1 and PD-1 in a cohort of 96 paraffin-embedded NPC samples and confirmed that AFAP1-AS1 and PD-1 are co-expressed in infiltrating lymphocytes in NPC tissue. Moreover, patients with high expression of AFAP1-AS1 or PD-1 in infiltrating lymphocytes were more prone to distant metastasis, and NPC patients with positive expression of both AFAP1-AS1 and PD-1 had the poorest prognosis. This study suggests that AFAP1-AS1 and PD-1 may be potential therapeutic targets in NPC and that patients with co-expression of AFAP1-AS1 and PD-1 may be ideal candidates for future clinical trials of anti-PD-1 immune therapy.
Mechanistic insight into the regioselectivity of Pd(ii)-catalyzed C-H functionalization of N-methoxy cinnamamide.[Pubmed:28379232]
Dalton Trans. 2017 Apr 19;46(16):5288-5296.
Computational studies have been applied to gain insight into the mechanism of Pd(ii) catalyzed alpha-C-H functionalization of N-methoxy cinnamamide. The results show that the whole catalytic cycle proceeds via sequential six steps, including (i) catalyst Pd(t-BuNC)2 oxidation with O2, (ii) O-H deprotonation, (iii) t-BuNC migratory insertion to the Pd-C bond, (iv) acyl migration, (v) C-H activation and (vi) reductive elimination. The regioselectivity for different C-H activation sites depends on the coordination structures of alpha-C or beta-C to the palladium(ii) center. The coordination of alpha-C to the palladium(ii) center shows a regular planar quadrilateral structure, which is stable. However, the beta-C coordinating to the palladium(ii) center mainly exhibits a distorted quadrilateral structure, which is relatively unstable. Thus, the barrier of alpha-C-H activation is much lower than that of beta-C-H activation. The present results provide a deep understanding of the site-selectivity of C-H activation.
Three 1D cyanide-bridged M(Ni, Pd, Pt)-Mn(II) Coordination Polymer: Synthesis, Crystal Structure and Magnetic Properties.[Pubmed:28380238]
Acta Chim Slov. 2017 Mac;64(1):215-220.
Three tetracyanide-containing building blocks K2[M(CN)4] (M = Ni, Pd, Pt) and one semi-closed macrocycle seven-coordinated manganese(II) compound have been employed to assemble cyanide-bridged heterometallic complexes, resulting in three cyanide-bridged MII-MnII complexes: [Mn(L)][Ni(CN)4] . 2H2O (1) [Mn(L)][Pd(CN)4] (2) and [Mn(L)][Pt(CN)4] (3) (L = 2,6-bis[1-(2-(N-methylamino)ethylimino)ethyl]pyridine). Single-crystal X-ray diffraction analysis shows their similar one-dimensional structure consisting of the alternating [Mn(L)]2+ species and [M(CN)4]2- building blocks, generating a cyanide-bridged neutral polymeric chain. In all three isostructural complexes the coordination geometry of manganese ion is a slightly distorted pentagonal-bipyramidal with the two cyanide nitrogen atoms at the trans positions and N5 coordinating mode at the equatorial plane from ligand L. Investigation over magnetic properties of these complexes reveals very weak antiferromagnetic interaction between neighboring Mn(II) ions bridged by the long NC-M-CN unit. A best-fit to the magnetic susceptibility of complexes 1-3 leads to the magnetic coupling constant of J = -0.081, -0.103 and -0.14 cm-1, respectively.
Pd-Catalyzed Autotandem Reactions with N-Tosylhydrazones. Synthesis of Condensed Carbo- and Heterocycles by Formation of a C-C Single Bond and a C horizontal lineC Double Bond on the Same Carbon Atom.[Pubmed:28379703]
Org Lett. 2017 Apr 21;19(8):2034-2037.
A new Pd-catalyzed autotandem reaction is introduced that consists of the cross-coupling of a benzyl bromide with a N-tosylhydrazone followed by an intramolecular Heck reaction with an aryl bromide. During the process, a single and a double C-C bond are formed on the same carbon atom. Two different arrangements for the reactive functional groups are possible, rendering great flexibility to the transformation. The same strategy led to 9-methylene-9H-fluorenes, 9-methylene-9H-xanthenes, 9-methylene-9,10-dihydroacridines, and also dihydropyrroloisoquinoline and dihydroindoloisoquinoline derivatives.
Enhanced 15-HPETE production during oxidant stress induces apoptosis of endothelial cells.[Pubmed:15967159]
Prostaglandins Other Lipid Mediat. 2005 May;76(1-4):19-34.
Oxidant stress plays an important role in the etiology of vascular diseases by increasing rates of endothelial cell apoptosis, but few data exist on the mechanisms involved. Using a unique model of oxidative stress based on selenium deficiency (-Se), the effects of altered eicosanoid production on bovine aortic endothelial cells (BAEC) apoptosis was evaluated. Oxidant stress significantly increased the immediate oxygenation product of arachidonic acid metabolized by the 15-lipoxygenase pathway, 15-hydroxyperoxyeicosatetraenoic acid (15-HPETE). Treatment of -Se BAEC with TNFalpha/cyclohexamide (CHX) exhibited elevated levels of apoptosis, which was significantly reduced by the addition of a specific 15-lipoxygenase inhibitor PD146176. Furthermore, the addition of 15-HPETE to PD146176-treated BAEC, partially restored TNF/CHX-induced apoptosis. Increased exposure to 15-HPETE induced apoptosis, as determined by internucleosomal DNA fragmentation, chromatin condensation, caspase-3 activation, and caspase-9 activation, which suggests mitochondrial dysfunction. The expression of Bcl-2 protein also was decreased in -Se BAEC. Addition of a caspase-9 inhibitor (LEHD-fmk) completely blocked 15-HPETE-induced chromatin condensation in -Se BAEC, suggesting that 15-HPETE-induced apoptosis is caspase-9 dependent. Increased apoptosis of BAEC as a result of oxidant stress and subsequent production of 15-HPETE may play a critical role in a variety of inflammatory based diseases.
A specific 15-lipoxygenase inhibitor limits the progression and monocyte-macrophage enrichment of hypercholesterolemia-induced atherosclerosis in the rabbit.[Pubmed:9543090]
Atherosclerosis. 1998 Feb;136(2):203-16.
Oxidant signalling and lipoprotein oxidation may play important roles in atherosclerotic lesion development. Given coincident localization of 15-lipoxygenase (15-LO), stereospecific products of 15-LO and epitopes of modified LDL in atherosclerotic lesions, we hypothesized that inhibition of 15-LO by PD146176, an inhibitor of 15-LO with an IC50 in cells or isolated enzyme of 0.5-0.8 microM, may limit atherosclerotic lesion development through regulation of monocyte-macrophage enrichment. Rabbits exposed to chronic endothelial denudation of the iliac-femoral artery were meal-fed a 0.25% cholesterol (C), 3% peanut oil (PNO), 3% coconut oil (CNO) diet twice daily with and without 175 mg/kg PD146176 for 12 weeks. In a second study, atherosclerotic lesions were pre-established in rabbits through chronic endothelial denudation and meal-fed a 0.5% C, 3% PNO, 3% CNO diet for 9 weeks and a 0% C/fat diet for 6 weeks prior to an 8 week administration of PD146176 at 175 mg/kg, q.d. Plasma total and lipoprotein cholesterol exposure were similar in control and PD146176-treated animals in both studies but PD146176 increased plasma triglyceride exposure 2- to 4-fold. Plasma PD146176 concentrations ranged from 99 to 214 ng/ml at 2 h post-dose. In the progression study, the iliac-femoral monocyte-macrophage area was reduced 71%, cross-sectional lesion area was unchanged and cholesteryl ester (CE) content was reduced 63%. In the regression study, size and macrophage content of iliac-femoral, fibrous plaque-like lesions were decreased 34%, CE content was reduced 19% and gross extent of thoracic aortic lesions were reduced 41%. We conclude that PD146176 can limit monocyte macrophage enrichment of atherosclerotic lesions and can attenuate development of fibrofoamy and fibrous plaque lesions in the absence of changes in plasma total or lipoprotein cholesterol concentrations.
Attenuation of diet-induced atherosclerosis in rabbits with a highly selective 15-lipoxygenase inhibitor lacking significant antioxidant properties.[Pubmed:9105693]
Br J Pharmacol. 1997 Apr;120(7):1199-206.
1. 15-Lipoxygenase (15-LO) has been implicated in the pathogenesis of atherosclerosis because of its localization in lesions and the many biological activities exhibited by its products. To provide further evidence for a role of 15-LO, the effects of PD 146176 on the development of atherosclerosis in cholesterol-fed rabbits were assessed. This novel drug is a specific inhibitor of the enzyme in vitro and lacks significant non specific antioxidant properties. 2. PD 146176 inhibited rabbit reticulocyte 15-LO through a mixed noncompetitive mode with a Ki of 197 nM. The drug had minimal effects on either copper or 2,2'-azobis(2-amidinopropane)hydrochloride (ABAP) induced oxidation of LDL except at concentrations 2 orders higher than the Ki. 3. Control New Zealand rabbits were fed a high-fat diet containing 0.25% wt./wt. cholesterol; treated animals received inhibitor in this diet (175 mg kg-1, b.i.d.). Plasma concentrations of inhibitor were similar to the estimated Ki (197 nM). During the 12 week study, there were no significant differences in weight gain haematocrit, plasma total cholesterol concentrations, or distribution of lipoprotein cholesterol. 4. The drug plasma concentrations achieved in vivo did not inhibit low-density lipoprotein (LDL) oxidation in vitro. Furthermore, LDL isolated from PD 146176-treated animals was as susceptible as that from controls to oxidation ex vivo by either copper or ABAP. 5. PD 146176 was very effective in suppressing atherogenesis, especially in the aortic arch where lesion coverage diminished from 15 +/- 4 to 0% (P < 0.02); esterified cholesterol content was reduced from 2.1 +/- 0.7 to 0 micrograms mg-1 (P < 0.02) in this region. Immunostainable lipid-laden macrophages present in aortic intima of control animals were totally absent in the drug-treated group. 6. Results of these studies are consistent with a role for 15-LO in atherogenesis.


