Lansoprazole sodiumPPI/cytochrome P450 inhibitor CAS# 226904-00-3 |
- Tenofovir
Catalog No.:BCC2500
CAS No.:147127-20-6
- Tenofovir Disoproxil Fumarate
Catalog No.:BCC1108
CAS No.:202138-50-9
- Entecavir Hydrate
Catalog No.:BCC1109
CAS No.:209216-23-9
- Lersivirine
Catalog No.:BCC1698
CAS No.:473921-12-9
- Didanosine
Catalog No.:BCC3763
CAS No.:69655-05-6
Quality Control & MSDS
Number of papers citing our products

Chemical structure

3D structure
| Cas No. | 226904-00-3 | SDF | Download SDF |
| PubChem ID | 12044540 | Appearance | Powder |
| Formula | C16H13F3N3NaO2S | M.Wt | 391.34 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in DMSO | ||
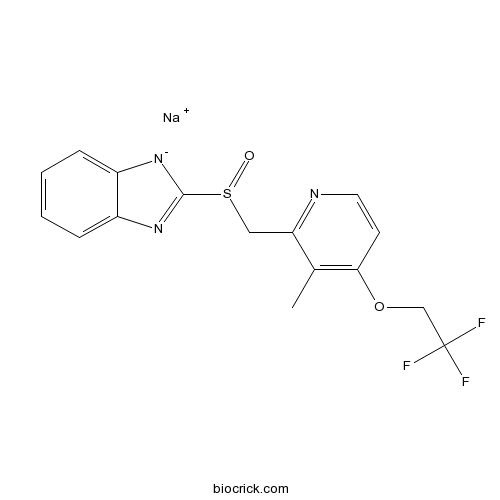
| Chemical Name | sodium;2-[[3-methyl-4-(2,2,2-trifluoroethoxy)pyridin-2-yl]methylsulfinyl]benzimidazol-1-ide | ||
| SMILES | CC1=C(C=CN=C1CS(=O)C2=NC3=CC=CC=C3[N-]2)OCC(F)(F)F.[Na+] | ||
| Standard InChIKey | OTGPYEHFRKGRIZ-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C16H13F3N3O2S.Na/c1-10-13(20-7-6-14(10)24-9-16(17,18)19)8-25(23)15-21-11-4-2-3-5-12(11)22-15;/h2-7H,8-9H2,1H3;/q-1;+1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Lansoprazole sodium Dilution Calculator

Lansoprazole sodium Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.5553 mL | 12.7766 mL | 25.5532 mL | 51.1065 mL | 63.8831 mL |
| 5 mM | 0.5111 mL | 2.5553 mL | 5.1106 mL | 10.2213 mL | 12.7766 mL |
| 10 mM | 0.2555 mL | 1.2777 mL | 2.5553 mL | 5.1106 mL | 6.3883 mL |
| 50 mM | 0.0511 mL | 0.2555 mL | 0.5111 mL | 1.0221 mL | 1.2777 mL |
| 100 mM | 0.0256 mL | 0.1278 mL | 0.2555 mL | 0.5111 mL | 0.6388 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Lansoprazole sodium is a proton-pump inhibitor (PPI) and also can inhibit cytochrome P450 [1].
Lansoprazole sodium is a substituted benzimidazole and has been widely used as acid inhibitory agent for treatment of disorders related to gastric acid secretion. Lansoprazole sodium has been exhibited to inhibit stomach acid by blocking the H+/K+ATPase system at the secretory surface of gastric parietal cells. As the human P450 enzymes including CYP2C9,2C19, 2D6 and 3A4, in addition, Lansoprazole sodium has been reported to have the competitively inhibitory effect on the activity of CYP2C9 in HLM with a Ki value of 21μM. Lansoprazole sodium has been found to be potent CYP2C19 inhibitor with a Ki value of about 1μM in both HLM and rCYP2C19 tests. Besides, Lansoprazole sodium has been revealed to inhibit the activities if CYP2D6 and CYP3A4 with IC50 values of both>200μM [1].
References:
[1] Li XQ1, Andersson TB, Ahlström M, Weidolf L.Comparison of inhibitory effects of the proton pump-inhibiting drugs omeprazole, esomeprazole, lansoprazole, pantoprazole, and rabeprazole on human cytochrome P450 activities. Drug Metab Dispos. 2004 Aug;32(8):821-7.
- Quercetin-3-O-glucuronide
Catalog No.:BCN3149
CAS No.:22688-79-5
- Kaempferol-3-beta-O-glucuronide
Catalog No.:BCN2503
CAS No.:22688-78-4
- NPS 2390
Catalog No.:BCC6119
CAS No.:226878-01-9
- 2-(3,4-dihydroxyphenyl)-2-hydroxypropanoic acid
Catalog No.:BCN6296
CAS No.:22681-72-7
- Bakkenolide Db
Catalog No.:BCN7117
CAS No.:226711-23-5
- Fosamprenavir Calcium Salt
Catalog No.:BCC1581
CAS No.:226700-81-8
- 3-Hydroxy-12-oleanene-23,28-dioic acid
Catalog No.:BCN1482
CAS No.:226562-47-6
- Amaronol B
Catalog No.:BCN5071
CAS No.:226561-02-0
- Amaronol A
Catalog No.:BCN5070
CAS No.:226560-96-9
- 4,6,7-Trihydroxycoumarin
Catalog No.:BCC9203
CAS No.:22649-24-7
- Torachrysone
Catalog No.:BCN5069
CAS No.:22649-04-3
- Cinacalcet
Catalog No.:BCC1483
CAS No.:226256-56-0
- 4-Hydroxy-1,10-secocadin-5-ene-1,10-dione
Catalog No.:BCN6661
CAS No.:226904-40-1
- LB42708
Catalog No.:BCC5344
CAS No.:226929-39-1
- Emapunil
Catalog No.:BCC5521
CAS No.:226954-04-7
- 3,6-Dimethoxyapigenin
Catalog No.:BCN4830
CAS No.:22697-65-0
- Hyponine E
Catalog No.:BCC8999
CAS No.:226975-99-1
- 9-Deacetyl-9-benzoyl-10-debenzoyl-4beta,20-epoxytaxchinin A
Catalog No.:BCN7676
CAS No.:227011-48-5
- AZ 11645373
Catalog No.:BCC7646
CAS No.:227088-94-0
- (3S,7S)-5,6-Dehydro-4''-de-O-methylcentrolobine
Catalog No.:BCN1481
CAS No.:227289-51-2
- Mucrolidin
Catalog No.:BCN5072
CAS No.:227471-20-7
- Xanthobaccin A
Catalog No.:BCN1864
CAS No.:227596-81-8
- Rubroside G
Catalog No.:BCN1856
CAS No.:227597-42-4
- Rubroside H
Catalog No.:BCN1857
CAS No.:227597-43-5
The effect of sequential therapy with lansoprazole and ecabet sodium in treating iatrogenic gastric ulcer after endoscopic submucosal dissection: a randomized prospective study.[Pubmed:25420889]
J Dig Dis. 2015 Feb;16(2):75-82.
OBJECTIVE: Ecabet sodium (ES) is a new non-systemic anti-ulcer agent belonging to the category of gastroprotective agents. In this study we aimed to compare the efficacy of a combination therapy with lansoprazole (LS) followed by ES with LS alone in treating endoscopic submucosal dissection (ESD)-induced iatrogenic gastric ulcers. METHODS: Patients diagnosed with gastric adenomas or early gastric cancer were randomly divided into either the LS group (30 mg once daily for 4 weeks; n = 45) or the LS + ES group (LS 30 mg once daily for one week followed by ES 1500 mg twice daily for 3 weeks; n = 45). Four weeks after ESD, a follow-up endoscopy was conducted to evaluate the proportions of ulcer reduction and ulcer stages in the two groups. RESULTS: In all, 79 patients were included in the final analyses. Both treatment modalities were well-tolerated in most patients, with a drug compliance of over 80%. There were no significant differences between the two groups in terms of the proportions of ulcer reduction (0.9503 +/- 0.1215 in the LS group vs 0.9192 +/- 0.0700 in the LS + ES group, P = 0.169) or ulcer stage (P = 0.446). The prevalence of adverse events related to drugs and bleeding were also similar between the two groups. CONCLUSION: Sequential therapy with LS + ES is as effective as LS alone against ESD-induced gastric ulcers.
Influences of sodium carbonate on physicochemical properties of lansoprazole in designed multiple coating pellets.[Pubmed:20717759]
AAPS PharmSciTech. 2010 Sep;11(3):1287-93.
Lansoprazole (LSP), a proton-pump inhibitor, belongs to class II drug. It is especially instable to heat, light, and acidic media, indicating that fabrication of a formulation stabilizing the drug is difficult. The addition of alkaline stabilizer is the most powerful method to protect the drug in solid formulations under detrimental environment. The purpose of the study was to characterize the designed multiple coating pellets of LSP containing an alkaline stabilizer (sodium carbonate) and assess the effect of the stabilizer on the physicochemical properties of the drug. The coated pellets were prepared by layer-layer film coating with a fluid-bed coater. In vitro release and acid-resistance studies were carried out in simulated gastric fluid and simulated intestinal fluid, respectively. Furthermore, the moisture-uptake test was performed to evaluate the influence of sodium carbonate on the drug stability. The results indicate that the drug exists in the amorphous state or small (nanometer size) particles without crystallization even after storage at 40 degrees C/75% for 5 months. The addition of sodium carbonate to the pellet protects the drug from degradation in simulated gastric fluid in a dose-dependent manner. The moisture absorbed into the pellets has a detrimental effect on the drug stability. The extent of drug degradation is directly correlated with the content of moisture absorption. In conclusion, these results suggest that the presence of sodium carbonate influence the physicochemical properties of LSP, and the designed multiple coating pellets enhance the drug stability.
A randomized, crossover pharmacodynamic study of immediate-release omeprazole/sodium bicarbonate and delayed-release lansoprazole in healthy adult volunteers.[Pubmed:27433347]
Pharmacol Res Perspect. 2016 May 19;4(3):e00238.
Proton pump inhibitors (PPIs) effectively block gastric acid secretion and are the treatment of choice for heartburn. PPIs differ, however, in onset of action and bioavailability. In this single-center, open-label, three-way crossover study, onset of action of immediate-release omeprazole 20 mg/sodium bicarbonate 1100 mg (IR-OME) and delayed-release (DR) lansoprazole 15 mg was evaluated in 63 healthy fasting adults. Subjects were randomized to once daily IR-OME, or DR-lansoprazole, or no treatment for 7 days. The primary efficacy endpoint was the earliest time where a statistically significant difference was observed between IR-OME and DR-lansoprazole in median intragastric pH scores for three consecutive 5-min intervals on day 7. Secondary endpoints compared effects of active treatments on days 1 and 7 (e.g., time to sustained inhibition, percentage of time with pH >4). A significant difference in median intragastric pH favoring IR-OME was observed on day 7 starting at the 10- to 15-min interval postdosing (P = 0.024) and sustaining through the 115- to 120-min interval (P = 0.017). On day 1, IR-OME achieved sustained inhibition of intragastric acidity significantly faster than DR-lansoprazole. IR-OME maintained pH >4 significantly longer than DR-lansoprazole over a 24-h period (P = 0.007) on day 7. Overall, results of this study demonstrate IR-OME is safe and well tolerated and that treatment with IR-OME results in significantly faster onset of action and better gastric acid suppression at steady state than DR-lansoprazole.
Triple therapy with ecabet sodium, amoxicillin and lansoprazole for 2 weeks as the rescue regimen for H. pylori infection.[Pubmed:21372444]
Intern Med. 2011;50(5):369-74. Epub 2011 Mar 1.
BACKGROUND/AIM: Ecabet sodium has an anti-H. pylori effect. We assessed the efficacy of ecabet sodium in the rescue therapy for the eradication of H. pylori. METHODS: A total of 74 patients with failed eradication of H. pylori after triple therapy with lansoprazole 30 mg bid, amoxicillin 750 mg bid and clarithromycin 200 mg bid were enrolled. They were randomly assigned to the three treatment groups as follows: LAC, lansoprazole 30 mg + amoxicillin 750 mg + clarithromycin 200 mg bid for 1 week; LAC2E, lansoprazole 30 mg bid + amoxicillin 750 mg bid + clarithromycin 200 mg bid + ecabet sodium 2 g bid for 1 week; and LA2E, lansoprazole 30 mg bid + amoxicillin 750 mg bid + ecabet sodium 2 g bid for 2 weeks. Eradication of H. pylori was assessed by the 13C-urea breath test after treatment. RESULTS: Eradication rates in intention-to-treat and per-protocol analyses were 20.0% (95% CI: 6.8-40.7) and 20.0% (6.8-40.7) with LAC, respectively, and 16.0% (4.5-36.1) and 17.4% (5.0-38.8) with LAC2E. In contrast, respective rates with LA2E were 75% (53.3-90.2) and 85.7% (63.7-97.0), which were significantly higher than those with LAC (p<0.001 for both ITT and PP) and LAC2E (p<0.001 for both ITT and PP). CONCLUSION: Triple therapy with ecabet sodium, lansoprazole and amoxicillin for 2 weeks was effective as the rescue therapy after failure of the standard clarithromycin-based regimen.


