LestaurtinibJAK2/FLT3/TrkA inhibitor CAS# 111358-88-4 |
- Nevirapine
Catalog No.:BCC3820
CAS No.:129618-40-2
- Lamivudine
Catalog No.:BCC3801
CAS No.:134678-17-4
- Delavirdine
Catalog No.:BCC4300
CAS No.:136817-59-9
- Emtricitabine
Catalog No.:BCC3774
CAS No.:143491-57-0
- Tenofovir
Catalog No.:BCC2500
CAS No.:147127-20-6
- Delavirdine mesylate
Catalog No.:BCC4069
CAS No.:147221-93-0
Quality Control & MSDS
Number of papers citing our products

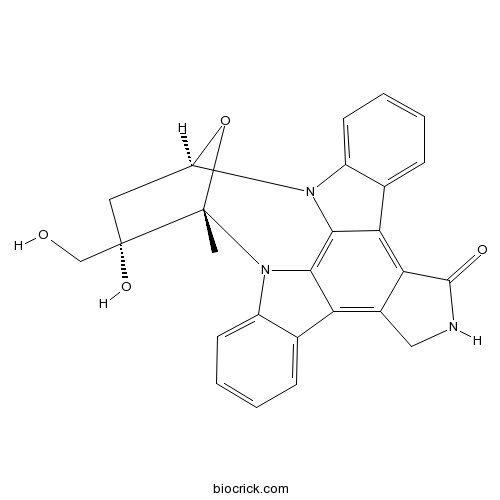
Chemical structure
3D structure
| Cas No. | 111358-88-4 | SDF | Download SDF |
| PubChem ID | 126565 | Appearance | Powder |
| Formula | C25H21N3O4 | M.Wt | 427.5 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in DMSO > 10 mM | ||
| SMILES | CC12C(CC(O1)N3C4=CC=CC=C4C5=C6C(=C7C8=CC=CC=C8N2C7=C53)CNC6=O)(CO)O | ||
| Standard InChIKey | UIARLYUEJFELEN-LROUJFHJSA-N | ||
| Standard InChI | InChI=1S/C26H21N3O4/c1-25-26(32,12-30)10-18(33-25)28-16-8-4-2-6-13(16)20-21-15(11-27-24(21)31)19-14-7-3-5-9-17(14)29(25)23(19)22(20)28/h2-9,18,30,32H,10-12H2,1H3,(H,27,31)/t18-,25+,26+/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Potent JAK2, FLT3 and TrkA inhibitor (IC50 values are 0.9, 3 and < 25 nM, respectively). Also inhibits Aurora kinase A and B (IC50 values are 8.1 and 2.3 nM, respectively) and prevents STAT5 phosphorylation (IC50 = 20 - 30 nM). Exhibits antiproliferative activity in vitro (IC50 = 30 - 100 nM in HEL92.1.7 cells) and is effective against myeloproliferative disorders in vivo. |

Lestaurtinib Dilution Calculator

Lestaurtinib Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.3392 mL | 11.6959 mL | 23.3918 mL | 46.7836 mL | 58.4795 mL |
| 5 mM | 0.4678 mL | 2.3392 mL | 4.6784 mL | 9.3567 mL | 11.6959 mL |
| 10 mM | 0.2339 mL | 1.1696 mL | 2.3392 mL | 4.6784 mL | 5.848 mL |
| 50 mM | 0.0468 mL | 0.2339 mL | 0.4678 mL | 0.9357 mL | 1.1696 mL |
| 100 mM | 0.0234 mL | 0.117 mL | 0.2339 mL | 0.4678 mL | 0.5848 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Potent JAK2, FLT3 and TrkA inhibitor (IC50 values are 0.9, 3 and < 25 nM respectively) that prevents STAT5 phosphorylation (IC50 = 20 - 30 nM). Exhibits antiproliferative activity in vitro (IC50 = 30 - 100 nM in HEL92.1.7 cells) and is effective against myeloproliferative disorders in vivo.
- Sappanol
Catalog No.:BCN3735
CAS No.:111254-19-4
- Episappanol
Catalog No.:BCN7940
CAS No.:111254-18-3
- CGS 20625
Catalog No.:BCC7375
CAS No.:111205-55-1
- 7,3',4'-Trihydroxy-3-benzyl-2H-chromene
Catalog No.:BCN1621
CAS No.:1111897-60-9
- 1,2-O-Dilinoleoyl-3-O-beta-D-galactopyranosylracglycerol
Catalog No.:BCN6768
CAS No.:111187-15-6
- PF-04880594
Catalog No.:BCC3998
CAS No.:1111636-35-1
- NF 110
Catalog No.:BCC7404
CAS No.:111150-22-2
- 14-Hydroxy sprengerinin C
Catalog No.:BCN2777
CAS No.:1111088-89-1
- FERb 033
Catalog No.:BCC7701
CAS No.:1111084-78-6
- Dacomitinib (PF299804, PF299)
Catalog No.:BCC3683
CAS No.:1110813-31-4
- Fmoc-Ser(Trt)-OH
Catalog No.:BCC3546
CAS No.:111061-56-4
- Fmoc-D-Lys(Trt)-OH
Catalog No.:BCC2594
CAS No.:111061-54-2
- Zileuton
Catalog No.:BCC2515
CAS No.:111406-87-2
- Azadirachtin
Catalog No.:BCC8123
CAS No.:11141-17-6
- Pinocembrin diacetate
Catalog No.:BCN5997
CAS No.:111441-88-4
- Axinysone B
Catalog No.:BCN7713
CAS No.:1114491-60-9
- Naltrindole hydrochloride
Catalog No.:BCC6773
CAS No.:111469-81-9
- Amlodipine Besylate
Catalog No.:BCC4397
CAS No.:111470-99-6
- 25-Anhydroalisol F
Catalog No.:BCN3361
CAS No.:1114895-01-0
- Ac-DL-Met-OH
Catalog No.:BCC2999
CAS No.:1115-47-5
- H-Ala-OEt.HCl
Catalog No.:BCC2687
CAS No.:1115-59-9
- L-Cysteinesulfinic acid
Catalog No.:BCC6571
CAS No.:1115-65-7
- Metformin HCl
Catalog No.:BCC4799
CAS No.:1115-70-4
- Nyasicoside
Catalog No.:BCN5998
CAS No.:111518-94-6
Phase I dose escalation study of lestaurtinib in patients with myelofibrosis.[Pubmed:25563429]
Leuk Lymphoma. 2015;56(9):2543-51.
We performed a multicenter, investigator initiated, phase I dose escalation study of the oral multi-kinase inhibitor Lestaurtinib in patients with JAK2V617F positive myelofibrosis, irrespective of baseline platelet count. A total of 34 patients were enrolled. Dose-limiting toxicities were observed in three patients overall, at the 100 mg (n = 1) and 160 mg (n = 2) twice-daily dose levels. The maximum tolerated dose was 140 mg twice daily. Gastrointestinal toxicity was the most common adverse event. Sixteen patients were evaluable for response at 12 weeks. Seven patients had clinical improvement by International Working Group - Myeloproliferative Neoplasms Research and Treatment criteria. Meaningful reductions in JAK2V617F allele burden were not observed. To measure JAK2 inhibition in vivo, plasma from treated patients was assayed for its ability to inhibit phosphorylation of signal transducer and activator of transcription 5 (STAT5): doses lower than 140 mg had variable and incomplete inhibition. In this phase I study, although gastrointestinal adverse events were common, significant clinical activity with Lestaurtinib was observed (ClinicalTrials.gov identifier: NCT00668421).
NGF-induced TrkA/CD44 association is involved in tumor aggressiveness and resistance to lestaurtinib.[Pubmed:25840418]
Oncotarget. 2015;6(12):9807-19.
There is accumulating evidence that TrkA and its ligand Nerve Growth Factor (NGF) are involved in cancer development. Staurosporine derivatives such as K252a and Lestaurtinib have been developed to block TrkA kinase signaling, but no clinical trial has fully demonstrated their therapeutic efficacy. Therapeutic failures are likely due to the existence of intrinsic signaling pathways in cancer cells that impede or bypass the effects of TrkA tyrosine kinase inhibitors. To verify this hypothesis, we combined different approaches including mass spectrometry proteomics, co-immunoprecipitation and proximity ligation assays. We found that NGF treatment induced CD44 binding to TrkA at the plasma membrane and subsequent activation of the p115RhoGEF/RhoA/ROCK1 pathway to stimulate breast cancer cell invasion. The NGF-induced CD44 signaling was independent of TrkA kinase activity. Moreover, both TrkA tyrosine kinase inhibition with Lestaurtinib and CD44 silencing with siRNA inhibited cell growth in vitro as well as tumor development in mouse xenograft model; combined treatment significantly enhanced the antineoplastic effects of either treatment alone. Altogether, our results demonstrate that NGF-induced tyrosine kinase independent TrkA signaling through CD44 was sufficient to maintain tumor aggressiveness. Our findings provide an alternative mechanism of cancer resistance to Lestaurtinib and indicate that dual inhibition of CD44 and TrkA tyrosine kinase activity may represent a novel therapeutic strategy.
A randomized assessment of adding the kinase inhibitor lestaurtinib to first-line chemotherapy for FLT3-mutated AML.[Pubmed:27872058]
Blood. 2017 Mar 2;129(9):1143-1154.
The clinical benefit of adding FMS-like tyrosine kinase-3 (FLT3)-directed small molecule therapy to standard first-line treatment of acute myeloid leukemia (AML) has not yet been established. As part of the UK AML15 and AML17 trials, patients with previously untreated AML and confirmed FLT3-activating mutations, mostly younger than 60 years, were randomly assigned either to receive oral Lestaurtinib (CEP701) or not after each of 4 cycles of induction and consolidation chemotherapy. Lestaurtinib was commenced 2 days after completing chemotherapy and administered in cycles of up to 28 days. The trials ran consecutively. Primary endpoints were overall survival in AML15 and relapse-free survival in AML17; outcome data were meta-analyzed. Five hundred patients were randomly assigned between Lestaurtinib and control: 74% had FLT3-internal tandem duplication mutations, 23% FLT3-tyrosine kinase domain point mutations, and 2% both types. No significant differences were seen in either 5-year overall survival (Lestaurtinib 46% vs control 45%; hazard ratio, 0.90; 95% CI 0.70-1.15; P = .3) or 5-year relapse-free survival (40% vs 36%; hazard ratio, 0.88; 95% CI 0.69-1.12; P = .3). Exploratory subgroup analysis suggested survival benefit with Lestaurtinib in patients receiving concomitant azole antifungal prophylaxis and gemtuzumab ozogamicin with the first course of chemotherapy. Correlative studies included analysis of in vivo FLT3 inhibition by plasma inhibitory activity assay and indicated improved overall survival and significantly reduced rates of relapse in Lestaurtinib-treated patients who achieved sustained greater than 85% FLT3 inhibition. In conclusion, combining Lestaurtinib with intensive chemotherapy proved feasible in younger patients with newly diagnosed FLT3-mutated AML, but yielded no overall clinical benefit. The improved clinical outcomes seen in patients achieving sustained FLT3 inhibition encourage continued evaluation of FLT3-directed therapy alongside front-line AML treatment. The UK AML15 and AML17 trials are registered at www.isrctn.com/ISRCTN17161961 and www.isrctn.com/ISRCTN55675535 respectively.
Enhancing SHP-1 expression with 5-azacytidine may inhibit STAT3 activation and confer sensitivity in lestaurtinib (CEP-701)-resistant FLT3-ITD positive acute myeloid leukemia.[Pubmed:26547689]
BMC Cancer. 2015 Nov 7;15:869.
BACKGROUND: Tumor-suppressor genes are inactivated by methylation in several cancers including acute myeloid leukemia (AML). Src homology-2 (SH2)-containing protein-tyrosine phosphatase 1 (SHP-1) is a negative regulator of the JAK/STAT pathway. Transcriptional silencing of SHP-1 plays a critical role in the development and progression of cancers through STAT3 activation. 5-Azacytidine (5-Aza) is a DNA methyltransferase inhibitor that causes DNA demethylation resulting in re-expression of silenced SHP-1. Lestaurtinib (CEP-701) is a multi-targeted tyrosine kinase inhibitor that potently inhibits FLT3 tyrosine kinase and induces hematological remission in AML patients harboring the internal tandem duplication of the FLT3 gene (FLT3-ITD). However, the majority of patients in clinical trials developed resistance to CEP-701. Therefore, the aim of this study, was to assess the effect of re-expression of SHP-1 on sensitivity to CEP-701 in resistant AML cells. METHODS: Resistant cells harboring the FLT3-ITD were developed by overexposure of MV4-11 to CEP-701, and the effects of 5-Aza treatment were investigated. Apoptosis and cytotoxicity of CEP-701 were determined using Annexin V and MTS assays, respectively. Gene expression was performed by quantitative real-time PCR. STATs activity was examined by western blotting and the methylation profile of SHP-1 was studied using MS-PCR and pyrosequencing analysis. Repeated-measures ANOVA and Kruskal-Wallis tests were used for statistical analysis. RESULTS: The cytotoxic dose of CEP-701 on resistant cells was significantly higher in comparison with parental and MV4-11R-cep + 5-Aza cells (p = 0.004). The resistant cells showed a significant higher viability and lower apoptosis compared with other cells (p < 0.001). Expression of SHP-1 was 7-fold higher in MV4-11R-cep + 5-Aza cells compared to parental and resistant cells (p = 0.011). STAT3 was activated in resistant cells. Methylation of SHP-1 was significantly decreased in MV4-11R-cep + 5-Aza cells (p = 0.002). CONCLUSIONS: The restoration of SHP-1 expression induces sensitivity towards CEP-701 and could serve as a target in the treatment of AML. Our findings support the hypothesis that, the tumor-suppressor effect of SHP-1 is lost due to epigenetic silencing and its re-expression might play an important role in re-inducing sensitivity to TKIs. Thus, SHP-1 is a plausible candidate for a role in the development of CEP-701 resistance in FLT3-ITD+ AML patients.


