ML 190CAS# 1355244-02-8 |
- BMN-673 8R,9S
Catalog No.:BCC1422
CAS No.:1207456-00-5
- XAV-939
Catalog No.:BCC1120
CAS No.:284028-89-3
- PJ34
Catalog No.:BCC1865
CAS No.:344458-19-1
- ABT-888 (Veliparib)
Catalog No.:BCC1267
CAS No.:912444-00-9
- Veliparib dihydrochloride
Catalog No.:BCC2076
CAS No.:912445-05-7
Quality Control & MSDS
Number of papers citing our products

Chemical structure

3D structure
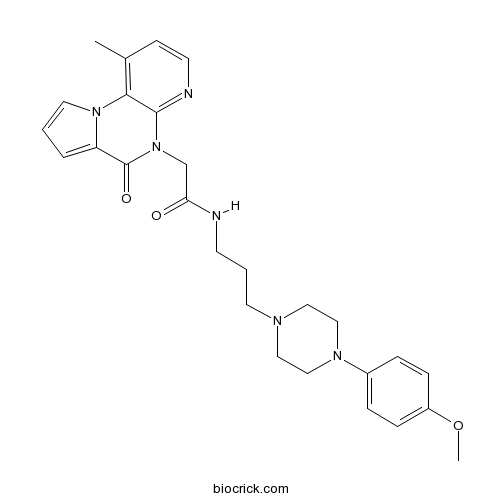
| Cas No. | 1355244-02-8 | SDF | Download SDF |
| PubChem ID | 44665680 | Appearance | Powder |
| Formula | C27H32N6O3 | M.Wt | 488.58 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 20 mM in DMSO | ||
| SMILES | CC1=C2C(=NC=C1)N(C(=O)C3=CC=CN32)CC(=O)NCCCN4CCN(CC4)C5=CC=C(C=C5)OC | ||
| Standard InChIKey | PMTIWRPLQBVEMR-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C27H32N6O3/c1-20-10-12-29-26-25(20)32-14-3-5-23(32)27(35)33(26)19-24(34)28-11-4-13-30-15-17-31(18-16-30)21-6-8-22(36-2)9-7-21/h3,5-10,12,14H,4,11,13,15-19H2,1-2H3,(H,28,34) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Selective κ opioid receptor (KOP) antagonist (IC50 = 120 nM in a β-arrestin assay); displays >267-fold selectivity over μ and δ opioid receptors. |

ML 190 Dilution Calculator

ML 190 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.0467 mL | 10.2337 mL | 20.4675 mL | 40.935 mL | 51.1687 mL |
| 5 mM | 0.4093 mL | 2.0467 mL | 4.0935 mL | 8.187 mL | 10.2337 mL |
| 10 mM | 0.2047 mL | 1.0234 mL | 2.0467 mL | 4.0935 mL | 5.1169 mL |
| 50 mM | 0.0409 mL | 0.2047 mL | 0.4093 mL | 0.8187 mL | 1.0234 mL |
| 100 mM | 0.0205 mL | 0.1023 mL | 0.2047 mL | 0.4093 mL | 0.5117 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- CYM 50260
Catalog No.:BCC6259
CAS No.:1355026-60-6
- Cabazitaxel intermediate
Catalog No.:BCN7432
CAS No.:1354900-65-4
- Araliadiol
Catalog No.:BCC8835
CAS No.:1354638-93-9
- Strontium Ranelate
Catalog No.:BCC3858
CAS No.:135459-87-9
- Rehmapicrogenin
Catalog No.:BCN8507
CAS No.:135447-39-1
- 7-O-Prenylscopoletin
Catalog No.:BCN3547
CAS No.:13544-37-1
- JW 480
Catalog No.:BCC6142
CAS No.:1354359-53-7
- trans-Ned 19
Catalog No.:BCC7825
CAS No.:1354235-96-3
- ACT 335827
Catalog No.:BCC6346
CAS No.:1354039-86-3
- KH CB19
Catalog No.:BCC6135
CAS No.:1354037-26-5
- Bullatine A
Catalog No.:BCN2374
CAS No.:1354-84-3
- CX-6258 hydrochloride hydrate
Catalog No.:BCC1505
CAS No.:1353858-99-7
- 2,3-Dihydro-12,13-dihydroxyeuparin
Catalog No.:BCN7189
CAS No.:135531-75-8
- Mutant IDH1-IN-1
Catalog No.:BCC6403
CAS No.:1355326-21-4
- AGI-5198
Catalog No.:BCC2293
CAS No.:1355326-35-0
- Cudraxanthone L
Catalog No.:BCN6187
CAS No.:135541-40-1
- Schisanlignone A
Catalog No.:BCN3628
CAS No.:135557-67-4
- Nav1.7 inhibitor
Catalog No.:BCC4191
CAS No.:1355631-24-1
- Pneumocandin B0
Catalog No.:BCC5436
CAS No.:135575-42-7
- Parsonsianidine
Catalog No.:BCN2109
CAS No.:135601-81-9
- Fmoc-D-His(Trt)-OH
Catalog No.:BCC3504
CAS No.:135610-90-1
- Coccineone B
Catalog No.:BCN6464
CAS No.:135626-13-0
- CP 80633
Catalog No.:BCC7463
CAS No.:135637-46-6
- 17-Methylparsonsianidine
Catalog No.:BCN2093
CAS No.:135637-68-2
MRI-detection rate and incidence of lumbar bleeding sources in 190 patients with non-aneurysmal SAH.[Pubmed:28369075]
PLoS One. 2017 Apr 3;12(4):e0174734.
BACKGROUND: Up to 15% of all spontaneous subarachnoid hemorrhages (SAH) have a non-aneurysmal SAH (NASAH). The evaluation of SAH patients with negative digital subtraction angiography (DSA) is sometimes a diagnostic challenge. Our goal in this study was to reassess the yield of standard MR-imaging of the complete spinal axis to rule out spinal bleeding sources in patients with NASAH. METHODS: We retrospectively analyzed the spinal MRI findings in 190 patients with spontaneous NASAH, containing perimesencephalic (PM) and non-perimesencephalic (NPM) SAH, diagnosed by computer tomography (CT) and/or lumbar puncture (LP), and negative 2nd DSA. RESULTS: 190 NASAH patients were included in the study, divided into PM-SAH (n = 87; 46%) and NPM-SAH (n = 103; 54%). Overall, 23 (22%) patients had a CT negative SAH, diagnosed by positive LP. MR-imaging of the spinal axis detected two patients with lumbar ependymoma (n = 2; 1,05%). Both patients complained of radicular sciatic pain. The detection rate raised up to 25%, if only patients with radicular sciatic pain received an MRI. CONCLUSION: Routine radiological investigation of the complete spinal axis in NASAH patients is expensive and can not be recommended for standard procedure. However, patients with clinical signs of low-back/sciatic pain should be worked up for a spinal pathology.
Use of Moderate-Intensity Statins for Low-Density Lipoprotein Cholesterol Level above 190 mg/dL at Baseline in Koreans.[Pubmed:28165667]
Basic Clin Pharmacol Toxicol. 2017 Oct;121(4):272-278.
The ACC/AHA 2013 guideline recommends high-intensity statin therapy for a decrease in low-density lipoprotein cholesterol (LDL-C) level by >50% among patients with baseline values of >/=190 mg/dL (approximately 4.872 mmol/L); however, this value should be modified before applying it to Korean populations. We investigated the statin-specific LDL-C-lowering effects in Korean patients with baseline LDL-C value >/=4.872 mmol/L. Data of patients prescribed a statin for the first time from January 2009 to December 2013 were assessed. In patients with baseline LDL-C value >/=4.872 mmol/L, laboratory data for a maximum of 6 months from the date of first statin prescription were collected. Among 33,721 patients who were prescribed a statin for the first time, 655 patients had a baseline LDL-C value >/=4.872 mmol/L (1.9%). Of these, 179 patients were analysed. Patients receiving moderate-intensity statins were divided into two groups based on LDL-C reduction rate (p = 0.0002), defined as moderate-high-intensity (atorvastatin 20 mg, rosuvastatin 10 mg, simvastatin 20 mg) and moderate-low-intensity (atorvastatin 10 mg, pitavastatin 2 mg, pravastatin 40 mg) statin groups. LDL-C reduction rates did not significantly differ between the moderate-high- and high-intensity statin groups (p = 0.4895). We found that some moderate-intensity statins demonstrated a LDL-C-lowering effect of more than 50% in Korean patients with a baseline LDL-C value >/=4.872 mmol/L. Our results reflect the need of a large-scale, randomized, controlled trial on partial reclassification of statins for patients with baseline LDL-C value >/=4.872 mmol/L before adopting ACC/AHC guidelines in Korea.
Depression and physical health multimorbidity: primary data and country-wide meta-analysis of population data from 190 593 people across 43 low- and middle-income countries.[Pubmed:28374652]
Psychol Med. 2017 Sep;47(12):2107-2117.
BACKGROUND: Despite the known heightened risk and burden of various somatic diseases in people with depression, very little is known about physical health multimorbidity (i.e. two or more physical health co-morbidities) in individuals with depression. This study explored physical health multimorbidity in people with clinical depression, subsyndromal depression and brief depressive episode across 43 low- and middle-income countries (LMICs). METHOD: Cross-sectional, community-based data on 190 593 individuals from 43 LMICs recruited via the World Health Survey were analysed. Multivariable logistic regression analysis was done to assess the association between depression and physical multimorbidity. RESULTS: Overall, two, three and four or more physical health conditions were present in 7.4, 2.4 and 0.9% of non-depressive individuals compared with 17.7, 9.1 and 4.9% among people with any depressive episode, respectively. Compared with those with no depression, subsyndromal depression, brief depressive episode and depressive episode were significantly associated with 2.62, 2.14 and 3.44 times higher odds for multimorbidity, respectively. A significant positive association between multimorbidity and any depression was observed across 42 of the 43 countries, with particularly high odds ratios (ORs) in China (OR 8.84), Laos (OR 5.08), Ethiopia (OR 4.99), the Philippines (OR 4.81) and Malaysia (OR 4.58). The pooled OR for multimorbidity and depression estimated by meta-analysis across 43 countries was 3.26 (95% confident interval 2.98-3.57). CONCLUSIONS: Our large multinational study demonstrates that physical health multimorbidity is increased across the depression spectrum. Public health interventions are required to address this global health problem.
Burden of disease attributed to ambient PM2.5 and PM10 exposure in 190 cities in China.[Pubmed:28321701]
Environ Sci Pollut Res Int. 2017 Apr;24(12):11559-11572.
Particulate air pollution is becoming a serious public health concern in urban cities of China. Association of disability-adjusted life years (DALYs) and economic loss with air pollution-related health effects demand quantitative analysis for correctional measures in air quality. This study applies an epidemiology-based exposure-response function to obtain the quantitative estimate of health impact of particulate matter PM2.5 and PM10 across 190 cities of China during years 2014-2015. The annual average concentration of PM2.5 and PM10 is 57 +/- 18 mug/m(3) (ranging from 18 to 119 mug/m(3)) and 97.7 +/- 34.2 mug/m(3) (ranging from 33.5 to 252.8 mug/m(3)), respectively. Based on the present study, the total estimated annual premature mortality due to PM2.5 is 722,370 [95% confidence interval (CI) = 322,716-987,519], 79% of which accounts for adult cerebrovascular disease (stroke) and ischemic heart disease (IHD). The premature mortality in megacities is very high, such as Chongqing (25,162/year), Beijing (19,702/year), Shanghai (19,617/year), Tianjin (13,726/year), and Chengdu (12,356/year). PM10 pollution has caused 1,491,774 (95% CI = 972,770-1,960,303) premature deaths (age >30) in China. Further, 3,614,064 cases of chronic bronchitis (CB); 13,759,894 cases of asthma attack among all ages; 191,709 COPD-related hospital admission (HA) cases; 499,048 respiratory-related HA; 357,816 cerebrovascular HA; and 308,129 cardiovascular-related HA due to PM10 pollution have been estimated during 2014-2015. Chongqing, Beijing, Baoding, Tianjin, and Shijiazhuang are the top five contributors to pollution-related mortality, accounting for 3.10, 2.71, 2.49, 2.20, and 2.02%, respectively, of the total deaths caused by PM10 pollution. The total DALYs associated with PM2.5 and PM10 pollution in China is 7.2 and 20.66 million in 2014-2015, and mortality and chronic bronchitis shared about 93.3% of the total DALYs for PM10. During this period, the economic cost of health impact due to PM10 is approximately US$304,122 million, which accounts for about 2.94% of China's gross domestic product (GDP). Megacities are expected to contribute relatively more to the total costs. The present methodology could be used as a tool to help policy makers and pollution control board authorities, to further analyze costs and benefits of air pollution management programs in China.
Discovery of Small Molecule Kappa Opioid Receptor Agonist and Antagonist Chemotypes through a HTS and Hit Refinement Strategy.[Pubmed:22737280]
ACS Chem Neurosci. 2012 Mar 21;3(3):221-236.
Herein we present the outcome of a high throughput screening (HTS) campaign-based strategy for the rapid identification and optimization of selective and general chemotypes for both kappa (kappa) opioid receptor (KOR) activation and inhibition. In this program, we have developed potent antagonists (IC(50) < 120 nM) or agonists of high binding affinity (K(i) < 3 nM). In contrast to many important KOR ligands, the compounds presented here are highly modular, readily synthesized and, in most cases, achiral. The four new chemotypes hold promise for further development into chemical tools for studying the KOR or as potential therapeutic lead candidates.


