PYR-41inhibitor of Ubiquitin-Activating Enzyme (E1) CAS# 418805-02-4 |
- BS-181
Catalog No.:BCC1439
CAS No.:1092443-52-1
- CDK inhibitor II
Catalog No.:BCC1464
CAS No.:1269815-17-9
- CGP60474
Catalog No.:BCC1474
CAS No.:164658-13-3
- SNS-032 (BMS-387032)
Catalog No.:BCC1152
CAS No.:345627-80-7
- Dinaciclib (SCH727965)
Catalog No.:BCC3765
CAS No.:779353-01-4
- AT7519
Catalog No.:BCC2541
CAS No.:844442-38-2
Quality Control & MSDS
Number of papers citing our products

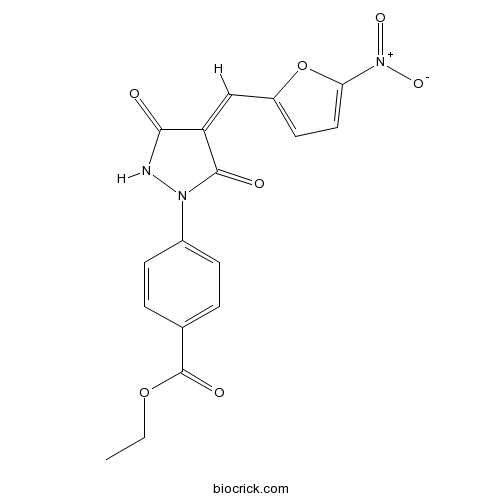
Chemical structure
3D structure
| Cas No. | 418805-02-4 | SDF | Download SDF |
| PubChem ID | 5335621 | Appearance | Powder |
| Formula | C17H13N3O7 | M.Wt | 371.3 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : ≥ 46 mg/mL (123.89 mM) *"≥" means soluble, but saturation unknown. | ||
| Chemical Name | ethyl 4-[(4Z)-4-[(5-nitrofuran-2-yl)methylidene]-3,5-dioxopyrazolidin-1-yl]benzoate | ||
| SMILES | CCOC(=O)C1=CC=C(C=C1)N2C(=O)C(=CC3=CC=C(O3)[N+](=O)[O-])C(=O)N2 | ||
| Standard InChIKey | ARGIPZKQJGFSGQ-LCYFTJDESA-N | ||
| Standard InChI | InChI=1S/C17H13N3O7/c1-2-26-17(23)10-3-5-11(6-4-10)19-16(22)13(15(21)18-19)9-12-7-8-14(27-12)20(24)25/h3-9H,2H2,1H3,(H,18,21)/b13-9- | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Cell-permeable, irreversible ubiquitin-activating enzyme (E1) inhibitor (IC50 < 10 μM). Blocks ubiquitylation and prevents ubiquitin-mediated proteasomal degradation. Inhibits NF-κB activation, blocks degradation of p53, increases p21 levels and induces apoptosis in vitro. Also causes an increase in sumoylation of proteins. |

PYR-41 Dilution Calculator

PYR-41 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.6932 mL | 13.4662 mL | 26.9324 mL | 53.8648 mL | 67.331 mL |
| 5 mM | 0.5386 mL | 2.6932 mL | 5.3865 mL | 10.773 mL | 13.4662 mL |
| 10 mM | 0.2693 mL | 1.3466 mL | 2.6932 mL | 5.3865 mL | 6.7331 mL |
| 50 mM | 0.0539 mL | 0.2693 mL | 0.5386 mL | 1.0773 mL | 1.3466 mL |
| 100 mM | 0.0269 mL | 0.1347 mL | 0.2693 mL | 0.5386 mL | 0.6733 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Ubiquitylation is catalyzed by the sequential action of ubiquitinactivating enzyme (E1), a ubiquitin-conjugating enzyme (E2) and a ubiquitin protein ligase (E3). Ubiquitylation is essential to numerous cellular and developmental processes, including protein quality control, growth, apoptosis, antigen presentation, DNA repair, and signal transduction. PYR-41, 4[4-(5-nitro-furan-2-ylmethylene)-3,5-dioxo-pyrazolidin-1-yl]-benzoic acid ethyl ester, is a selective inhibitor of Ubiquitin-Activating Enzyme (E1).
In vitro: In addition to blocking ubiquitylation, PYR-41 was found to increase total sumoylation in cells. PYR-41 could attenuate cytokine-mediated nuclear factor-KBactivation. This correlated with inhibition of nonproteasomal ubiquitylation of TRAF6, which is important to IKBkinase activation. PYR-41 also prevented the downstream ubiquitylation and proteasomal degradation of IKBA. Moreover, PYR-41 has demonstrated effective UAE E1 inhibition as well as some off-target inhibition of the other ubiquitin regulatory enzymes and signal-transducing proteins, suggesting it is a nonspecific inhibitor [1].
In vivo: No animal in vivo data have been published so far.
Clinical trial: Up to now, PYR-41 is still in the preclinical development stage.
Reference:
[1] Yang Y1 Kitagaki J, Dai RM, Tsai YC, Lorick KL, Ludwig RL, Pierre SA, Jensen JP, Davydov IV, Oberoi P, Li CC, Kenten JH, Beutler JA, Vousden KH, Weissman AM. Inhibitors of ubiquitin-activating enzyme (E1), a new class of potential cancer therapeutics. Cancer Res. 2007 Oct 1;67(19):9472-81.
- 2,3-DCPE hydrochloride
Catalog No.:BCC2384
CAS No.:418788-90-6
- Bezafibrate
Catalog No.:BCC4639
CAS No.:41859-67-0
- Alatamine
Catalog No.:BCN3100
CAS No.:41855-33-8
- Mangiferolic acid
Catalog No.:BCN4636
CAS No.:4184-34-3
- Hesperetin-7-methyl ether
Catalog No.:BCN8501
CAS No.:
- Junicedric acid
Catalog No.:BCN7660
CAS No.:41787-69-3
- Hypecorinine
Catalog No.:BCN3298
CAS No.:41787-57-9
- H-Leu-pNA.HCl
Catalog No.:BCC2972
CAS No.:4178-93-2
- Lenvatinib (E7080)
Catalog No.:BCC1172
CAS No.:417716-92-8
- 14-Deoxyandrographolide
Catalog No.:BCN3706
CAS No.:4176-97-0
- ar-Curcumene
Catalog No.:BCN7534
CAS No.:4176-06-1
- Evonimine
Catalog No.:BCN3082
CAS No.:41758-69-4
- Nitrocefin
Catalog No.:BCC6544
CAS No.:41906-86-9
- Isopropyl 4-Hydroxybenzoate
Catalog No.:BCN8409
CAS No.:4191-73-5
- Trilobatin
Catalog No.:BCN5479
CAS No.:4192-90-9
- N1-Methyl-4-nitrobenzene-1,2-diamine
Catalog No.:BCC9070
CAS No.:41939-61-1
- MLR 1023
Catalog No.:BCC6232
CAS No.:41964-07-2
- Pinocembrin chalcone
Catalog No.:BCN7223
CAS No.:4197-97-1
- Glabranin
Catalog No.:BCN5480
CAS No.:41983-91-9
- (S)-(-)-Propranolol hydrochloride
Catalog No.:BCC6809
CAS No.:4199-10-4
- 5-Chloro-1,10-phenanthroline
Catalog No.:BCC3713
CAS No.:4199-89-7
- Dihydropashanone
Catalog No.:BCN4635
CAS No.:41997-41-5
- Antitumor Compound 1
Catalog No.:BCC5397
CAS No.:420126-30-3
- Fenofibric acid
Catalog No.:BCC8982
CAS No.:42017-89-0
Galectin-9 induces atypical ubiquitination leading to cell death in PC-3 prostate cancer cells.[Pubmed:30383222]
Glycobiology. 2019 Jan 1;29(1):22-35.
Galectin-9 is the most potent inducer of cell death in lymphomas and other malignant cell types among the members of the galectin family. We investigated the mechanism of galectin-9-induced cell death in PC-3 prostate cancer cells in comparison with in Jurkat T cells. Galectin-9 induced apoptotic cell death in Jurkat cells, as typically revealed by DNA ladder formation. On the other hand, DNA ladder formation and other features of apoptosis were not apparent in PC-3 cells undergoing galectin-9-induced death. Exogenous galectin-9 was endocytosed and destined to the lysosomal compartment in PC-3 cells. The internalized galectin-9 was resistant to detergent solubilization but was solubilized with lactose. Agents inhibiting actin filament dynamics abolished the internalization and cytocidal effect of galectin-9 in PC-3 but not Jurkat cells. Galectin-9 induced accumulation of ubiquitinated proteins, possibly heterogeneously ubiquitinated and/or monoubiquitinated proteins, in PC-3 cells. PYR-41, an inhibitor of the ubiquitin-activating E1 enzyme, suppressed the cytocidal effect of galectin-9. Although ubiquitination was upregulated also in Jurkat cells by galectin-9, PYR-41 was ineffective against galectin-9-induced cell death. Colocalization of ubiquitinated proteins and LAMP-1 was detectable in PC-3 cells treated with galectin-9. The ubiquitinated proteins were recovered in the insoluble fraction upon cell fractionation. In contrast, ubiquitinated proteins that accumulated after treatment with proteasome inhibitors did not co-localize with LAMP-1 and were mainly recovered in soluble fraction. The results suggest that atypical ubiquitination and accumulation of ubiquitinated proteins in lysosomes play a pivotal role in galectin-9-induced non-apoptotic death in PC-3 cells.
Dynamic regulation of dynein localization revealed by small molecule inhibitors of ubiquitination enzymes.[Pubmed:30257893]
Open Biol. 2018 Sep 26;8(9). pii: rsob.180095.
Cytoplasmic dynein is a minus-end-directed microtubule-based motor that acts at diverse subcellular sites. During mitosis, dynein localizes simultaneously to the mitotic spindle, spindle poles, kinetochores and the cell cortex. However, it is unclear what controls the relative targeting of dynein to these locations. As dynein is heavily post-translationally modified, we sought to test a role for these modifications in regulating dynein localization. We find that dynein rapidly and strongly accumulates at mitotic spindle poles following treatment with NSC697923, a small molecule that inhibits the ubiquitin E2 enzyme, Ubc13, or treatment with PYR-41, a ubiquitin E1 inhibitor. Subsets of dynein regulators such as Lis1, ZW10 and Spindly accumulate at the spindle poles, whereas others do not, suggesting that NSC697923 differentially affects specific dynein populations. We additionally find that dynein relocalization induced by NSC697923 or PYR-41 can be suppressed by simultaneous treatment with the non-selective deubiquitinase inhibitor, PR-619. However, we did not observe altered dynein localization following treatment with the selective E1 inhibitor, TAK-243. Although it is possible that off-target effects of NSC697923 and PYR-41 are responsible for the observed changes in dynein localization, the rapid relocalization upon drug treatment highlights the highly dynamic nature of dynein regulation during mitosis.
Administration of ubiquitin-activating enzyme UBA1 inhibitor PYR-41 attenuates angiotensin II-induced cardiac remodeling in mice.[Pubmed:30249396]
Biochem Biophys Res Commun. 2018 Oct 20;505(1):317-324.
Pathological cardiac hypertrophy is the main risk factor for heart diseases. The ubiquitin-proteasome system (UPS) is the major intracellular protein degradation system involved in the development of cardiac hypertrophic remodeling. Ubiquitin-activating enzyme E1, a key component of the UPS, catalyzes the first step in ubiquitin conjugation to mark cellular proteins for degradation via proteasome. However, the functional role of E1 (UBA1) in regulation of hypertrophic remodeling in angiotensin II (Ang II)-infused mice remains unknown. In this study, male wild-type mice were treated with UBA1 inhibitor PYR-41at two doses of 5 and 10mg and infused with Ang II (1000ng/kg/min) for 14 days. Systolic blood pressure was detected by using tail-cuff system. Cardiac function was assessed by echocardiography. Hypertrophic remodeling was analyzed examined by histological examinations. The expressions of genes and proteins were detected by quantitative real-time PCR and immunoblotting analysis. After 14 days, Ang II infusion significantly increased UBA1 expression at both mRNA and protein levels in the hearts. Furthermore, Ang II-infused mice showed a significant increase in systolic blood pressure compensatory cardiac function, hypertrophy, interstitial fibrosis, inflammation and oxidative stress compared with saline-treated controls, whereas these effects were dose-dependently attenuated in PYR-41-treated mice. These beneficial actions were associated mainly with inhibition of PTEN degradation and multiple downstream mediators (AKT, ERK1/2, STAT3, TGF-beta/Smad2/3 and NF-kB(p65)). In conclusion, these results indicate that inhibition of UBA1 suppresses Ang II-induced hypertrophic remodeling, and suggest that administration of low dose PYR-41 may be a new potential therapeutic approach for treating hypertensive heart diseases.
PYR-41 and Thalidomide Impair Dendritic Cell Cross-Presentation by Inhibiting Myddosome Formation and Attenuating the Endosomal Recruitments of p97 and Sec61 via NF-kappaB Inactivation.[Pubmed:30069488]
J Immunol Res. 2018 Jul 5;2018:5070573.
PYR-41 and thalidomide have therapeutic effects on inflammation-associated diseases with side effects such as tumorigenesis. Cross-presentation allows dendritic cells (DC) to present endogenous antigen and induce protective immunity against microbe infection and tumors. But, up to now, the effects of PYR-41 and thalidomide on cross-presentation are still uncertain. In this study, we investigated the effect and mechanism of PYR-41 and thalidomide on DC cross-presentation by observing Myddosome formation, endosomal recruitment of p97 and Sec61, NF-kappaB activation, and cross-priming ability. We demonstrated that the inhibition of endosomal recruitment of p97 and Sec61, together with attenuated NF-kappaB activation and Myddosome formation, contributes to PYR-41- and thalidomide-impaired cross-presentation and thereby reverses cross-activation of T cells. These observations suggest that NF-kappaB signaling and p97 and Sec61 molecules are candidates for dealing with the side effects of PYR-41 and thalidomide.
Inhibition of ubiquitin-activating enzyme protects against organ injury after intestinal ischemia-reperfusion.[Pubmed:29771572]
Am J Physiol Gastrointest Liver Physiol. 2018 Aug 1;315(2):G283-G292.
Intestinal ischemia-reperfusion (I/R) occurs in various clinical settings, such as transplantation, acute mesenteric arterial occlusion, trauma, and shock. I/R injury causes severe systemic inflammation, leading to multiple organ dysfunction associated with high mortality. The ubiquitin proteasome pathway has been indicated in the regulation of inflammation, particularly through the NF-kappaB signaling pathway. PYR-41 is a small molecular compound that selectively inhibits ubiquitin-activating enzyme E1. A mouse model of intestinal I/R injury by clamping the superior mesenteric artery for 45 min was performed to evaluate the effect of PYR-41 treatment on organ injury and inflammation. PYR-41 was administered intravenously at the beginning of reperfusion. Blood and organ tissues were harvested at 4 h after reperfusion. PYR-41 treatment improved the morphological structure of gut and lung after I/R, as judged by hematoxylin and eosin staining. It also reduced the number of apoptotic terminal deoxynucleotidyl transferase dUTP nick end-labeling-positive cells and caspase-3 activity in the organs. PYR-41 treatment decreased the expression of proinflammatory cytokines IL-6 and IL-1beta as well as chemokines keratinocyte chemoattractant and macrophage inflammatory protein-2 in the gut and lung, which leads to inhibition of neutrophils infiltrating into these organs. The serum levels of IL-6, aspartate aminotransferase, and lactate dehydrogenase were reduced by the treatment. The IkappaB degradation in the gut increased after I/R was inhibited by PYR-41 treatment. Thus, ubiquitination may be a potential therapeutic target for treating patients suffering from intestinal I/R. NEW & NOTEWORTHY Excessive inflammation contributes to organ injury from intestinal ischemia-reperfusion (I/R) in many clinical conditions. NF-kappaB signaling is very important in regulating inflammatory response. In an experimental model of gut I/R injury, we demonstrate that administration of a pharmacological inhibitor of ubiquitination process attenuates NF-kappaB activation, leading to reduction of inflammation, tissue damage, and apoptosis in the gut and lungs. Therefore, ubiquitination process may serve as a therapeutic target for treating patients with intestinal I/R injury.
Lipopolysaccharide Mediates the Destruction of Intercellular Tight Junction among Renal Tubular Epithelial Cells via RhoT1/SMAD-4/JAM-3 Pathway.[Pubmed:29725250]
Int J Med Sci. 2018 Mar 14;15(6):595-602.
Background: The morbidity of sepsis induced acute kidney injury remains unacceptable high and the mechanisms of that disease remains unclear. For urine backleak and intercellular tight junction among tubular epithelial cells (TECs) destruction often occur during sepsis induced acute kidney injury, we examined whether lipopolysaccharide could damage intercellular tight junction among TECs and associated mechanisms in our present study. Methods: HK-2 cells were cultured, transfected with different SiRNAs and stimulated with LPS and PYR-41. Transepithelial Permeability Assay and Transepithelial Electrical Resistance Assay were used to evaluate intercellular tight junction destruction and Western Blot and Immunofluorescence were used to evaluate proteins expression. Results: Transepithelial Permeability increased significantly (P<0.05) and Transepithelial Electrical Resistance reduced remarkably (P<0.05) of the monolayer TECs stimulated with LPS. The expression of JAM-3 and RhoT1 decreased significantly (P<0.05) in TECs stimulated with LPS, while the level of SMAD-4 increased significantly (P<0.05). Downregulation of the expression of SMAD-4 with RNA interference could increase the expression of JAM-3 in LPS treated TECs. Moreover, upregulation of RhoT1 level by decreased the degradation of RhoT1 could decrease the expression of SMAD-4 and increase the JAM-3 level in TECs treated with LPS, while downregulation of RhoT1 level with RNA interference had the opposite effects. Conclusion: LPS mediates intercellular tight junction destruction among TECs and RhoT1/SMAD-4/JAM-3 is a pivotal pathway to mediate the phenomenon.
Targeting the SUMO pathway as a novel treatment for anaplastic thyroid cancer.[Pubmed:29383121]
Oncotarget. 2017 Oct 23;8(70):114801-114815.
Cancer stem cells (CSCs) are expanded in anaplastic thyroid cancer (ATC) and standard treatment approaches have failed to improve survival, suggesting a need to specifically target the CSC population. Recent studies in breast and colorectal cancer demonstrated that inhibition of the SUMO pathway repressed CD44 and cleared the CSC population, mediated through SUMO-unconjugated TFAP2A. We sought to evaluate effects of inhibiting the SUMO pathway in ATC. ATC cell lines and primary ATC tumor samples were evaluated. The SUMO pathway was inhibited by knockdown of PIAS1 and use of SUMO inhibitors anacardic acid and PYR-41. The expression of TFAP2A in primary ATC was examined by immunohistochemistry. All ATC cell lines expressed TFAP2A but only 8505C expressed SUMO-conjugated TFAP2A. In 8505C only, inhibition of the SUMO pathway by knockdown of PIAS1 or treatment with SUMO inhibitors repressed expression of CD44 with a concomitant loss of SUMO-conjugated TFAP2A. The effect of SUMO inhibition on CD44 expression was dependent upon TFAP2A. Treatment with SUMO inhibitors resulted in a statistically improved tumor-free survival in mice harboring 8505C xenografts. An examination of primary ATC tissue determined that TFAP2A was expressed in 4 of 11 tumors surveyed. We conclude that inhibition of the SUMO pathway repressed the CSC population, delaying the outgrowth of tumor xenografts in ATC. The effect of SUMO inhibition was dependent upon expression of SUMO-conjugated TFAP2A, which may serve as a molecular marker for therapeutic effects of SUMO inhibitors. The findings provide pre-clinical evidence for development of SUMO inhibitors for the treatment of ATC.
Ubiquitin-activating enzyme E1 inhibitor PYR-41 retards sperm enlargement after fusion to the egg.[Pubmed:29355596]
Reprod Toxicol. 2018 Mar;76:71-77.
The ubiquitin-proteasome system, which is initiated by a single ubiquitin-activating enzyme E1 (UBE1), is involved in male reproduction via spermatogenesis and function in mammals. Here we explored the influence of UBE1-specific inhibitor, 4[4-(5-nitro-furan-2-ylmethylene)-3,5-dioxo-pyrazolidin-1-yl]-benzoic acid ethyl ester (pyrazone-41 or PYR-41) in female reproduction. UBE-1 was detected by immunoblotting and immunocytochemistry in mouse eggs and was localized mainly under the egg plasma membrane. PYR-41 pretreatment suppresses the development of eggs into two-cell embryos. Specifically, pretreatment retarded sperm enlargement and meiotic chromosomal division after sperm-egg fusion. PYR-41 pretreatment disturbed beta-catenin, a well-known target protein for ubiquitination, localization under the egg plasma membrane and on spindle microtubules in wild-type eggs. Otherwise, PYR-41 treatment had no effect on the two-cell development of eggs lacking beta-catenin. Our results raise the possibility that inhibition of the ubiquitin-proteasome system suppresses sperm enlargement through impaired beta-catenin-mediated mechanism.
Proteasome-mediated degradation of collagen III by cortisol in amnion fibroblasts.[Pubmed:29191827]
J Mol Endocrinol. 2018 Feb;60(2):45-54.
Rupture of fetal membranes (ROM) can initiate parturition at both term and preterm. Collagen III in the compact layer of the amnion contributes to the tensile strength of fetal membranes. However, the upstream signals triggering collagen III degradation remain mostly elusive. In this study, we investigated the role of cortisol regenerated by 11beta-hydroxysteroid dehydrogenase 1 (11beta-HSD1) in collagen III degradation in human amnion fibroblasts with an aim to seek novel targets for the prevention of preterm premature ROM (pPROM)-elicited preterm birth. Human amnion tissue and cultured amnion tissue explants and amnion fibroblasts were used to study the regulation of collagen III, which is composed of three identical 3alpha 1 chains (COL3A1), by cortisol. Cortisol decreased COL3A1 protein but not mRNA abundance in a concentration-dependent manner. Cortisone also decreased COL3A1 protein, which was blocked by 11beta-HSD1 inhibition. The reduction in COL3A1 protein by cortisol was not affected by a transcription inhibitor but was further enhanced by a translation inhibitor. Autophagic pathway inhibitor chloroquine or siRNA-mediated knock-down of ATG7, an essential protein for autophagy, failed to block cortisol-induced reduction in COL3A1 protein abundance, whereas proteasome pathway inhibitors MG132 and bortezomib significantly attenuated cortisol-induced reduction in COL3A1 protein abundance. Moreover, cortisol increased COL3A1 ubiquitination and the reduction of COL3A1 protein by cortisol was blocked by PYR-41, a ubiquitin-activating enzyme inhibitor. Conclusively, cortisol regenerated in amnion fibroblasts may be associated with ROM at parturition by reducing collagen III protein abundance through a ubiquitin-proteasome pathway.
Nicotine increases apoptosis in HUVECs cultured in high glucose/high fat via Akt ubiquitination and degradation.[Pubmed:28963785]
Clin Exp Pharmacol Physiol. 2018 Feb;45(2):198-204.
It is well-documented that nicotine, the main active ingredient in cigarettes, results in endothelial cell injury in numerous diseases. However, whether nicotine plays a crucial role in endothelial cell injury in diabetes and the exact molecular mechanism that mediates this process have not been fully elucidated. The current study aimed to investigate the effects of nicotine on endothelial cell injury in diabetes and the specific molecular mechanism by which it plays a role. Human umbilical vein endothelial cells (HUVECs) were incubated in HG/HF media and treated with nicotine, PYR-41 (a selective ubiquitin E1 inhibitor), Akt-overexpressing adenovirus, or TTC3 and MUL1 shRNA adenovirus. Cell viability was subsequently detected by the CCK8 assay, and apoptosis was examined by caspase-3 cleavage and activity analysis. Compared to the HG/HF incubated group, nicotine incubation significantly decreased cell survival and increased apoptosis. Moreover, nicotine induced Akt degradation via UPS, and Akt overexpression blocked nicotine-induced apoptosis in HUVECs cultured in HG/HF media. Furthermore, the TTC3 and MUL1 shRNA adenovirus dramatically decreased the Akt ubiquitination and apoptosis induced by nicotine. These results indicate that nicotine-induced Akt ubiquitination and degradation occurs through TTC3 and MUL1 and results in a dramatic increase in apoptosis in HUVECs cultured in HG/HF media.
PYR-41, A Ubiquitin-Activating Enzyme E1 Inhibitor, Attenuates Lung Injury in Sepsis.[Pubmed:28661933]
Shock. 2018 Apr;49(4):442-450.
During sepsis, systemic inflammation is observed and is associated with multiple organ failure. Activation of NF-kappaB is crucial for inducing inflammation, which is controlled by degradation of inhibitor molecules (IkappaB). The ubiquitination proteasome pathway is responsible for the regulation of protein turnover. In this study, we hypothesized that administration of 4[4-(5-nitro-furan-2-ylmethylene)-3, -dioxo-pyrazolidin-1-yl]-benzoic acid ethyl ester (PYR-41), an inhibitor of ubiquitination, could reduce inflammation and organ injury in septic mice. PYR-41 prevented the reduction of IkappaB protein levels and inhibited release of tumor necrosis factor (TNF)-alpha in mouse macrophage RAW264.7 cells at 4 h after lipopolysaccharide stimulation dose-dependently. Male C57BL/6 mice were subjected to cecal ligation and puncture (CLP) to induce sepsis. PYR-41 (5 mg/kg) or dimethyl sulfoxide in saline (vehicle) was injected intravenously immediately after CLP. At 20 h after CLP, PYR-41 treatment significantly decreased serum levels of proinflammatory cytokines (TNF-alpha, interleukin [IL]-1beta, and IL-6) and organ injury markers (aspartate aminotransferase, alanine aminotransferase, and lactate dehydrogenase). PYR-41 significantly improved microscopic structure, and reduced myeloperoxidase activity, number of apoptotic cells and caspase-3 degradation in the lungs of septic mice. The reduced protein levels of IkappaB in the lungs after CLP were restored by PYR-41 treatment. PYR-41 inhibited the expression of cytokines (IL-1beta and IL-6), chemokines (keratinocyte-derived chemokine and macrophage inflammatory protein 2), and inflammatory mediators (cyclooxygenase-2 and inducible nitric oxide synthase) in the lungs of septic mice. Importantly, PYR-41 significantly increased 10-day survival in septic mice from 42% to 83%. Therefore, targeting ubiquitination by PYR-41 to inhibit NF-kappaB activation may represent a potential strategy of sepsis therapeutics.
PKC-alpha Triggers EGFR Ubiquitination, Endocytosis and ERK Activation in Podocytes Stimulated with High Glucose.[Pubmed:28535513]
Cell Physiol Biochem. 2017;42(1):281-294.
BACKGROUND: Protein Kinase C-alpha (PKC-alpha) and epidermal growth factor receptor (EGFR) are both involved in diabetic kidney disease; however, the connection between these two proteins during high glucose-induced podocyte injury remains uncertain. METHODS: Diabetes was induced in SD rats by streptozotocin (STZ). Fourteen days later, the kidney cortex was removed and subjected to plasma membrane isolation and lipid raft fractionation. In vitro study human podocyte cell line was differentiated and subjected to various treatments. The levels of membranous protein and endocytosis were assessed by biotinylation and sodium 2-mercaptoethane sulfonate (MesNa) treatment. Go6976 and PYR-41 were used as inhibitors of PKC-alpha and ubiquitin activating E1 enzyme, respectively. RESULTS: In diabetic rats, the abundance of PKC-alpha in the membranous fraction and the lipid raft domain is elevated, whereas the EGFR level is reduced. Consistently, in vitro high glucose treated podocytes, membranous EGFR is downregulated with increased PKC-alpha. Furthermore, the ubiquitination and endocytosis of EGFR are enhanced accompanied by extracellular signal-regulated kinase (ERK) signaling activation and podocyte damage during hyperglycemia. However, these processes can be ameliorated by inhibition of either PKC-alpha or ubiquitin activating E1 enzyme. CONCLUSION: During hyperglycemia, PKC-alpha mediates podocytic EGFR ubiquitination, endocytosis from cell surface and the subsequent ERK activation, which contributes to podocyte injury.
MDM2 mediates fibroblast activation and renal tubulointerstitial fibrosis via a p53-independent pathway.[Pubmed:28100501]
Am J Physiol Renal Physiol. 2017 Apr 1;312(4):F760-F768.
It is well recognized that murine double minute gene 2 (MDM2) plays a critical role in cell proliferation and inflammatory processes during tumorigenesis. It is also reported that MDM2 is expressed in glomeruli and involved in podocyte injury. However, whether MDM2 is implicated in renal fibrosis remains unclear. Here we investigated the role of MDM2 in tubulointerstitial fibrosis (TIF). By immunohistochemical staining and Western blotting we confirmed that MDM2 is upregulated in the tubulointerstitial compartment in patients with TIF and unilateral urethral obstruction (UUO) mice, which mainly originates from myofibroblasts. Consistently, in vitro MDM2 is increased in TGF-beta1-treated fibroblasts, one of the major sources of collagen-producing myofibroblasts during TIF, along with fibroblast activation. Importantly, genetic deletion of MDM2 significantly attenuates fibroblast activation. We then analyzed the possible downstream signaling of MDM2 during fibroblast activation. p53-dependent pathway is the classic downstream signaling of MDM2, and Nutlin-3 is a small molecular inhibitor of MDM2-p53 interaction. To our surprise, Nutlin-3 could not ameliorate fibroblast activation in vitro and TIF in UUO mice. However, we found that Notch1 signaling is attenuated during fibroblast activation, which could be markedly rescued by MDM2 knockdown. Overexpression of intracellular domain of Notch1 (NICD) by plasmid could obviously minimize fibroblast activation induced by TGF-beta1. In addition, the degradation of NICD is strikingly suppressed by PYR-41, an inhibitor of ubiquitin-activating enzyme E1, and proteasome inhibitor MG132. Taken together, our findings provide the first evidence that MDM2 is involved in fibroblast activation and TIF, which associates with Notch1 ubiquitination and proteasome degradation.
Increasing the Endoplasmic Reticulum Pool of the F508del Allele of the Cystic Fibrosis Transmembrane Conductance Regulator Leads to Greater Folding Correction by Small Molecule Therapeutics.[Pubmed:27732613]
PLoS One. 2016 Oct 12;11(10):e0163615.
Small molecules that correct the folding defects and enhance surface localization of the F508del mutation in the Cystic Fibrosis Transmembrane conductance Regulator (CFTR) comprise an important therapeutic strategy for cystic fibrosis lung disease. However, compounds that rescue the F508del mutant protein to wild type (WT) levels have not been identified. In this report, we consider obstacles to obtaining robust and therapeutically relevant levels of F508del CFTR. For example, markedly diminished steady state amounts of F508del CFTR compared to WT CFTR are present in recombinant bronchial epithelial cell lines, even when much higher levels of mutant transcript are present. In human primary airway cells, the paucity of Band B F508del is even more pronounced, although F508del and WT mRNA concentrations are comparable. Therefore, to augment levels of "repairable" F508del CFTR and identify small molecules that then correct this pool, we developed compound library screening protocols based on automated protein detection. First, cell-based imaging measurements were used to semi-quantitatively estimate distribution of F508del CFTR by high content analysis of two-dimensional images. We evaluated ~2,000 known bioactive compounds from the NIH Roadmap Molecular Libraries Small Molecule Repository in a pilot screen and identified agents that increase the F508del protein pool. Second, we analyzed ~10,000 compounds representing diverse chemical scaffolds for effects on total CFTR expression using a multi-plate fluorescence protocol and describe compounds that promote F508del maturation. Together, our findings demonstrate proof of principle that agents identified in this fashion can augment the level of endoplasmic reticulum (ER) resident "Band B" F508del CFTR suitable for pharmacologic correction. As further evidence in support of this strategy, PYR-41-a compound that inhibits the E1 ubiquitin activating enzyme-was shown to synergistically enhance F508del rescue by C18, a small molecule corrector. Our combined results indicate that increasing the levels of ER-localized CFTR available for repair provides a novel route to correct F508del CFTR.
Trichostatin A, a histone deacetylase inhibitor suppresses NADPH Oxidase 4-Derived Redox Signalling and Angiogenesis.[Pubmed:27297729]
J Cell Mol Med. 2016 Oct;20(10):1932-44.
Histone deacetylase (HDAC) inhibitors are known to suppress abnormal development of blood vessels. Angiogenic activity in endothelial cells depends upon NADPH oxidase 4 (Nox4)-dependent redox signalling. We set out to study whether the HDAC inhibitor trichostatin A (TSA) affects Nox4 expression and angiogenesis. Nox4 expression was measured by real time PCR and Western blot analysis in endothelial cells. Hydrogen peroxide (H2 O2 ) was measured by amplex((R)) red assay in endothelial cells. Nox4 was knocked down by Nox4 shRNA. In vitro angiogenic activities such migration and tubulogenesis were assessed using wound healing and Matrigel assays, respectively. In vivo angiogenic activity was assessed using subcutaneous sponge assay in C57Bl/6 and Nox4-deficient mice. Trichostatin A reduced Nox4 expression in a time- and concentration-dependent manner. Both TSA and Nox4 silencing decreased Nox4 protein and H2 O2 . Mechanistically, TSA reduced expression of Nox4 via ubiquitination of p300- histone acetyltransferase (p300-HAT). Thus, blocking of the ubiquitination pathway using an inhibitor of ubiquitin-activating enzyme E1 (PYR-41) prevented TSA inhibition of Nox4 expression. Trichostatin A also reduced migration and tube formation, and these effects were not observed in Nox4-deficient endothelial cells. Finally, transforming growth factor beta1 (TGFbeta1) enhanced angiogenesis in sponge model in C57BL/6 mice. This response to TGFbeta1 was substantially reduced in Nox4-deficient mice. Similarly intraperitoneal infusion of TSA (1 mg/kg) also suppressed TGFbeta1-induced angiogenesis in C57BL/6 mice. Trichostatin A reduces Nox4 expression and angiogenesis via inhibition of the p300-HAT-dependent pathway. This mechanism might be exploited to prevent aberrant angiogenesis in diabetic retinopathy, complicated vascular tumours and malformations.
Homology Modelling of Human E1 Ubiquitin Activating Enzyme.[Pubmed:20396627]
Lett Drug Des Discov. 2010 Jan 1;7(1):57-62.
Human E1 is a key player in protein ubiquitination, however the E1 structure is not available. In this paper, we describe the derivation of a human E1 structure using molecular modelling based on the crystal structure of S. cerevisiae E1 and M. Musculus E1. Key interactions between our E1 model and ubiquitin are also discussed.
Cancer preventive isothiocyanates induce selective degradation of cellular alpha- and beta-tubulins by proteasomes.[Pubmed:19339240]
J Biol Chem. 2009 Jun 19;284(25):17039-51.
Although it is conceivable that cancer preventive isothiocyanates (ITCs), a family of compounds in cruciferous vegetables, induce cell cycle arrest and apoptosis through a mechanism involving oxidative stress, our study shows that binding to cellular proteins correlates with their potencies of apoptosis induction. More recently, we showed that ITCs bind selectively to tubulins. The differential binding affinities toward tubulin among benzyl isothiocyanate, phenethyl isothiocyanate, and sulforaphane correlate well with their potencies of inducing tubulin conformation changes, microtubule depolymerization, and eventual cell cycle arrest and apoptosis in human lung cancer A549 cells. These results support that tubulin binding by ITCs is an early event for cell growth inhibition. Here we demonstrate that ITCs can selectively induce degradation of both alpha- and beta-tubulins in a variety of human cancer cell lines in a dose- and time-dependent manner. The onset of degradation, a rapid and irreversible process, is initiated by tubulin aggregation, and the degradation is proteasome-dependent. Results indicate that the degradation is triggered by ITC binding to tubulin and is irrelevant to oxidative stress. This is the first report that tubulin, a stable and abundant cytoskeleton protein required for cell cycle progression, can be selectively degraded by a small molecule.
Inhibitors of ubiquitin-activating enzyme (E1), a new class of potential cancer therapeutics.[Pubmed:17909057]
Cancer Res. 2007 Oct 1;67(19):9472-81.
The conjugation of proteins with ubiquitin plays numerous regulatory roles through both proteasomal-dependent and nonproteasomal-dependent functions. Alterations in ubiquitylation are observed in a wide range of pathologic conditions, including numerous malignancies. For this reason, there is great interest in targeting the ubiquitin-proteasome system in cancer. Several classes of proteasome inhibitors, which block degradation of ubiquitylated proteins, are widely used in research, and one, Bortezomib, is now in clinical use. Despite the well-defined and central role of the ubiquitin-activating enzyme (E1), no cell permeable inhibitors of E1 have been identified. Such inhibitors should, in principle, block all functions of ubiquitylation. We now report 4[4-(5-nitro-furan-2-ylmethylene)-3,5-dioxo-pyrazolidin-1-yl]-benzoic acid ethyl ester (PYR-41) as the first such inhibitor. Unexpectedly, in addition to blocking ubiquitylation, PYR-41 increased total sumoylation in cells. The molecular basis for this is unknown; however, increased sumoylation was also observed in cells harboring temperature-sensitive E1. Functionally, PYR-41 attenuates cytokine-mediated nuclear factor-kappaB activation. This correlates with inhibition of nonproteasomal (Lys-63) ubiquitylation of TRAF6, which is essential to IkappaB kinase activation. PYR-41 also prevents the downstream ubiquitylation and proteasomal degradation of IkappaBalpha. Furthermore, PYR-41 inhibits degradation of p53 and activates the transcriptional activity of this tumor suppressor. Consistent with this, it differentially kills transformed p53-expressing cells. Thus, PYR-41 and related pyrazones provide proof of principle for the capacity to differentially kill transformed cells, suggesting the potential for E1 inhibitors as therapeutics in cancer. These inhibitors can also be valuable tools for studying ubiquitylation.


