Paeonilactone ACAS# 98751-79-2 |
Quality Control & MSDS
Number of papers citing our products

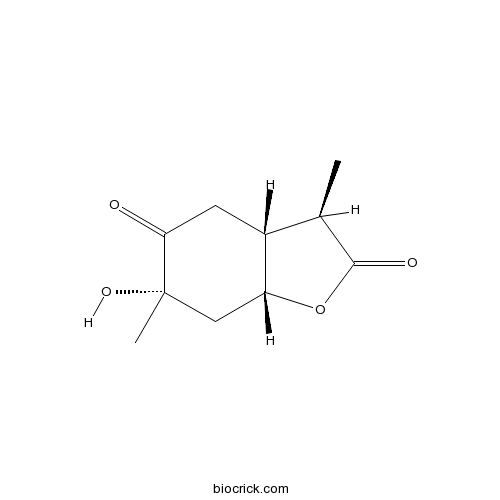
Chemical structure

3D structure
| Cas No. | 98751-79-2 | SDF | Download SDF |
| PubChem ID | 10081437 | Appearance | Powder |
| Formula | C10H14O4 | M.Wt | 198.2 |
| Type of Compound | Monoterpenoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | (3R,3aR,6S,7aR)-6-hydroxy-3,6-dimethyl-3a,4,7,7a-tetrahydro-3H-1-benzofuran-2,5-dione | ||
| SMILES | CC1C2CC(=O)C(CC2OC1=O)(C)O | ||
| Standard InChIKey | NODZICYHUGDVAM-IBNKKVAHSA-N | ||
| Standard InChI | InChI=1S/C10H14O4/c1-5-6-3-8(11)10(2,13)4-7(6)14-9(5)12/h5-7,13H,3-4H2,1-2H3/t5-,6-,7-,10+/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Standard reference |
| Structure Identification | J Org Chem. 2000 Apr 7;65(7):2122-6.An enantioselective route to paeonilactone A via palladium- and copper-catalyzed reactions.[Pubmed: 10774035]
Fitoterapia. 2007 Jan;78(1):76-8.Monoterpene glycosides from Paeonia delavayi.[Pubmed: 17067761]
|

Paeonilactone A Dilution Calculator

Paeonilactone A Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 5.0454 mL | 25.227 mL | 50.4541 mL | 100.9082 mL | 126.1352 mL |
| 5 mM | 1.0091 mL | 5.0454 mL | 10.0908 mL | 20.1816 mL | 25.227 mL |
| 10 mM | 0.5045 mL | 2.5227 mL | 5.0454 mL | 10.0908 mL | 12.6135 mL |
| 50 mM | 0.1009 mL | 0.5045 mL | 1.0091 mL | 2.0182 mL | 2.5227 mL |
| 100 mM | 0.0505 mL | 0.2523 mL | 0.5045 mL | 1.0091 mL | 1.2614 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Paeonilactone B
Catalog No.:BCN3963
CAS No.:98751-78-1
- Paeonilactone C
Catalog No.:BCN3964
CAS No.:98751-77-0
- Ropivacaine HCl
Catalog No.:BCC4841
CAS No.:98717-15-8
- ATP disodium salt
Catalog No.:BCC5160
CAS No.:987-65-5
- Dregeoside Ga1
Catalog No.:BCN4548
CAS No.:98665-66-8
- Dregeoside Da1
Catalog No.:BCN4764
CAS No.:98665-65-7
- Ganoderic acid G
Catalog No.:BCN2915
CAS No.:98665-22-6
- Ganolucidic acid A
Catalog No.:BCN2444
CAS No.:98665-21-5
- Ganoderic acid I
Catalog No.:BCN2865
CAS No.:98665-20-4
- Ganoderic acid H
Catalog No.:BCN3038
CAS No.:98665-19-1
- Lucidenic acid D2
Catalog No.:BCN8202
CAS No.:98665-16-8
- Ganoderic Acid J
Catalog No.:BCN8436
CAS No.:100440-26-4
- Latifoline N-oxide
Catalog No.:BCN1979
CAS No.:98752-06-8
- Isothymusin
Catalog No.:BCN4532
CAS No.:98755-25-0
- Reboxetine mesylate
Catalog No.:BCC4934
CAS No.:98769-84-7
- 3-(4-Hydroxy-3-methoxyphenyl)propyl tetracosanoate
Catalog No.:BCN1292
CAS No.:98770-70-8
- (R)-(+)-Bay K 8644
Catalog No.:BCC7107
CAS No.:98791-67-4
- FLAG tag Peptide
Catalog No.:BCC2562
CAS No.:98849-88-8
- Danshenxinkun D
Catalog No.:BCN2472
CAS No.:98873-76-8
- Pseudolaric acid B-O-beta-D-glucopyranoside
Catalog No.:BCN1291
CAS No.:98891-41-9
- Pseudolaric acid A-O-beta-D-glucopyranoside
Catalog No.:BCN1290
CAS No.:98891-44-2
- 3-Epiursolic acid
Catalog No.:BCN3173
CAS No.:989-30-0
- (-)-Epigallocatechin gallate
Catalog No.:BCN6326
CAS No.:989-51-5
- Limonol
Catalog No.:BCN4533
CAS No.:989-61-7
An enantioselective route to paeonilactone A via palladium- and copper-catalyzed reactions.[Pubmed:10774035]
J Org Chem. 2000 Apr 7;65(7):2122-6.
We herein report on a formal total synthesis of Paeonilactone A involving palladium-, copper-, and enzyme-catalyzed reactions starting from 1,3-cyclohexadiene. The key step in the synthesis, a palladium(II)-catalyzed 1,4-oxylactonization of a conjugated diene, simultaneously introduces two of the oxygen substituents required for the target molecule. The synthesis also includes our recently developed copper(I)-catalyzed cross-coupling reaction between dienyltriflates with Grignard reagents, introducing one of the methyl groups present in the target molecule. This new approach toward Paeonilactone A allows complete control of all four stereogenic centers and is the first enantioselective route toward Paeonilactone A starting from an achiral substrate.
Monoterpene glycosides from Paeonia delavayi.[Pubmed:17067761]
Fitoterapia. 2007 Jan;78(1):76-8.
A new monoterpene glycoside, 4-O-methyl-4''-hydroxy-3''-methoxy-paeoniflorin (1), was isolated from the root cortex of Paeonia delavayi along with the known paeoniflorin, oxypaeoniflorin, benzoylpaeoniflorin, benzoyloxypaeoniflorin, albiflorin and a paeonilactone-A.


