ProcaineCAS# 59-46-1 |
- Ibutilide Fumarate
Catalog No.:BCC5076
CAS No.:122647-32-9
- Amiloride HCl dihydrate
Catalog No.:BCC5068
CAS No.:17440-83-4
- Triamterene
Catalog No.:BCC5074
CAS No.:396-01-0
- Procaine HCl
Catalog No.:BCC5072
CAS No.:51-05-8
- Proparacaine HCl
Catalog No.:BCC5073
CAS No.:5875-06-9
- Phenytoin sodium
Catalog No.:BCC5071
CAS No.:630-93-3
Quality Control & MSDS
Number of papers citing our products

Chemical structure

3D structure
| Cas No. | 59-46-1 | SDF | Download SDF |
| PubChem ID | 4914 | Appearance | Powder |
| Formula | C13H20N2O2 | M.Wt | 236.31 |
| Type of Compound | Alkaloids | Storage | Desiccate at -20°C |
| Solubility | DMSO : 100 mg/mL (423.17 mM; Need ultrasonic) H2O : 1 mg/mL (4.23 mM; ultrasonic and warming and heat to 60°C) | ||
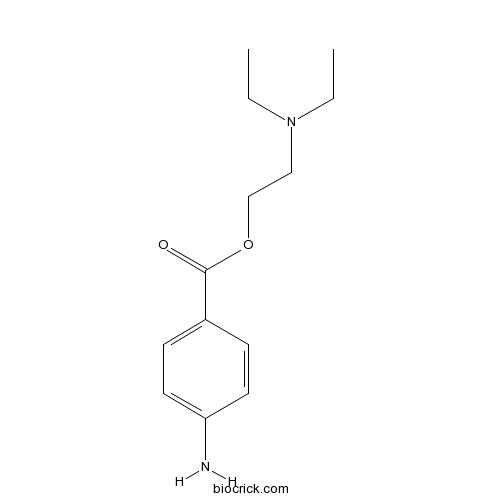
| Chemical Name | 2-(diethylamino)ethyl 4-aminobenzoate | ||
| SMILES | CCN(CC)CCOC(=O)C1=CC=C(C=C1)N | ||
| Standard InChIKey | MFDFERRIHVXMIY-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C13H20N2O2/c1-3-15(4-2)9-10-17-13(16)11-5-7-12(14)8-6-11/h5-8H,3-4,9-10,14H2,1-2H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Procaine is a benzoic acid derivative with local anesthetic and antiarrhythmic properties. Procaine binds to and inhibits voltage-gated sodium channels, thereby inhibiting the ionic flux required for the initiation and conduction of impulses. It is a DNA-demethylating agent with growth-inhibitory effects in human cancer cells, it inhibits the Wnt canonical pathway by promoter demethylation of WIF-1 in lung cancer cells. |
| Targets | DNA/RNA Synthesis |
| In vitro | Procaine is a DNA-demethylating agent with growth-inhibitory effects in human cancer cells.[Pubmed: 12941824]Cancer Res. 2003 Aug 15;63(16):4984-9.Methylation-associated silencing of tumor suppressor genes is recognized as being a molecular hallmark of human cancer. Unlike genetic alterations, changes in DNA methylation are potentially reversible. This possibility has attracted considerable attention from a therapeutics standpoint. Nucleoside-analogue inhibitors of DNA methyltransferases, such as 5-aza-2'-deoxycytidine, are able to demethylate DNA and restore silenced gene expression. Unfortunately, the clinical utility of these compounds has not yet been fully realized, mainly because of their side effects. A few non-nucleoside inhibitors of DNA methyltransferases have been reported, including the anti-arrhythmia drug procainamide. Following this need to find new demethylating agents, we have tested the potential use of Procaine, an anesthetic drug related to procainamide.
Procaine and mepivacaine have less toxicity in vitro than other clinically used local anesthetics.[Pubmed: 12818948]Anesth Analg. 2003 Jul;97(1):85-90,The neurotoxicity of local anesthetics can be demonstrated in vitro by the collapse of growth cones and neurites in cultured neurons. We compared the neurotoxicity of Procaine, mepivacaine, ropivacaine, bupivacaine, lidocaine, tetracaine, and dibucaine by using cultured neurons from the freshwater snail Lymnaea stagnalis. A solution of local anesthetics was added to the culture dish to make final concentrations ranging from 1 x 10(-6) to 2 x 10(-2) M. Morphological changes in the growth cones and neurites were observed and graded 1 (moderate) or 2 (severe). The median concentrations yielding a score of 1 were 5 x 10(-4) M for Procaine, 5 x 10(-4) M for mepivacaine, 2 x 10(-4) M for ropivacaine, 2 x 10(-4) M for bupivacaine, 1 x 10(-4) M for lidocaine, 5 x 10(-5) M for tetracaine, and 2 x 10(-5) M for dibucaine. Statistically significant differences (P < 0.05) were observed between mepivacaine and ropivacaine, bupivacaine and lidocaine, lidocaine and tetracaine, and tetracaine and dibucaine. The order of neurotoxicity was Procaine = mepivacaine < ropivacaine = bupivacaine < lidocaine < tetracaine < dibucaine. Although lidocaine is more toxic than bupivacaine and ropivacaine, mepivacaine, which has a similar pharmacological effect to lidocaine, has the least-adverse effects on cone growth among clinically used local anesthetics. |
| Kinase Assay | Procaine and procainamide inhibit the Wnt canonical pathway by promoter demethylation of WIF-1 in lung cancer cells.[Pubmed: 19885602]Oncol Rep. 2009 Dec;22(6):1479-84.Secreted Wingless type (Wnt) ligands have previously been shown to be involved in tumor developmental processes and oncogenesis. Aberrant promoter methylation of Wnt inhibitory factor-1 (WIF-1) is a fundamental mechanism of epigenetic silencing in human cancers. Procaine, a local anesthetic drug, and procainamide, a drug for the treatment of cardiac arrhythmias, have been reported as inhibitors of DNA methylation, causing demethylation and reactivation of methylation-silenced genes such as RARbeta and GSTP1. The promoter demethylation of WIF-1 has not previously been reported on. We demonstrated previously that WIF-1 is silenced due to promoter hypermethylation in lung cancer cell lines. |

Procaine Dilution Calculator

Procaine Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 4.2317 mL | 21.1586 mL | 42.3173 mL | 84.6346 mL | 105.7932 mL |
| 5 mM | 0.8463 mL | 4.2317 mL | 8.4635 mL | 16.9269 mL | 21.1586 mL |
| 10 mM | 0.4232 mL | 2.1159 mL | 4.2317 mL | 8.4635 mL | 10.5793 mL |
| 50 mM | 0.0846 mL | 0.4232 mL | 0.8463 mL | 1.6927 mL | 2.1159 mL |
| 100 mM | 0.0423 mL | 0.2116 mL | 0.4232 mL | 0.8463 mL | 1.0579 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Procaine is a local anesthetic drug of the amino ester group, which acts through multiple targets.
- Thiamine chloride
Catalog No.:BCN8344
CAS No.:59-43-8
- Sulfaquinoxaline
Catalog No.:BCC9158
CAS No.:59-40-5
- Mepyramine maleate
Catalog No.:BCC6740
CAS No.:59-33-6
- Folic acid
Catalog No.:BCN5375
CAS No.:59-30-3
- D-Galactose
Catalog No.:BCN8528
CAS No.:59-23-4
- 5-BrdU
Catalog No.:BCC5293
CAS No.:59-14-3
- Ethopabate
Catalog No.:BCC8964
CAS No.:59-06-3
- Methotrexate
Catalog No.:BCC2301
CAS No.:59-05-2
- DL-alpha-Tocopherol
Catalog No.:BCN2200
CAS No.:59-02-9
- Xanthurenic acid
Catalog No.:BCC7866
CAS No.:59-00-7
- Bestatin
Catalog No.:BCC1221
CAS No.:58970-76-6
- Cyclizine 2HCl
Catalog No.:BCC4518
CAS No.:5897-18-7
- Oxindole
Catalog No.:BCN4050
CAS No.:59-48-3
- DL-Methionine
Catalog No.:BCC8318
CAS No.:59-51-8
- Nicotinic acid
Catalog No.:BCN8328
CAS No.:59-67-6
- Nitrofurazone
Catalog No.:BCC3825
CAS No.:59-87-0
- Levodopa
Catalog No.:BCN1098
CAS No.:59-92-7
- Tolazoline HCl
Catalog No.:BCC4321
CAS No.:59-97-2
- Betaine hydrochloride
Catalog No.:BCN6304
CAS No.:590-46-5
- Bethanechol chloride
Catalog No.:BCC4566
CAS No.:590-63-6
- alpha-Endorphin
Catalog No.:BCC1010
CAS No.:59004-96-5
- 8-Hydroxyhyperforin 8,1-hemiacetal
Catalog No.:BCN4091
CAS No.:59014-02-7
- Atropine sulfate monohydrate
Catalog No.:BCC3728
CAS No.:5908-99-6
- Dehydrotoxicarol
Catalog No.:BCN3991
CAS No.:59086-93-0
Procaine and mepivacaine have less toxicity in vitro than other clinically used local anesthetics.[Pubmed:12818948]
Anesth Analg. 2003 Jul;97(1):85-90, table of contents.
UNLABELLED: The neurotoxicity of local anesthetics can be demonstrated in vitro by the collapse of growth cones and neurites in cultured neurons. We compared the neurotoxicity of Procaine, mepivacaine, ropivacaine, bupivacaine, lidocaine, tetracaine, and dibucaine by using cultured neurons from the freshwater snail Lymnaea stagnalis. A solution of local anesthetics was added to the culture dish to make final concentrations ranging from 1 x 10(-6) to 2 x 10(-2) M. Morphological changes in the growth cones and neurites were observed and graded 1 (moderate) or 2 (severe). The median concentrations yielding a score of 1 were 5 x 10(-4) M for Procaine, 5 x 10(-4) M for mepivacaine, 2 x 10(-4) M for ropivacaine, 2 x 10(-4) M for bupivacaine, 1 x 10(-4) M for lidocaine, 5 x 10(-5) M for tetracaine, and 2 x 10(-5) M for dibucaine. Statistically significant differences (P < 0.05) were observed between mepivacaine and ropivacaine, bupivacaine and lidocaine, lidocaine and tetracaine, and tetracaine and dibucaine. The order of neurotoxicity was Procaine = mepivacaine < ropivacaine = bupivacaine < lidocaine < tetracaine < dibucaine. Although lidocaine is more toxic than bupivacaine and ropivacaine, mepivacaine, which has a similar pharmacological effect to lidocaine, has the least-adverse effects on cone growth among clinically used local anesthetics. IMPLICATIONS: Systematic comparison was assessed morphologically in growth cones and neurites exposed to seven local anesthetics. The order of neurotoxicity was Procaine = mepivacaine < ropivacaine = bupivacaine < lidocaine < tetracaine < dibucaine. Although lidocaine is more toxic than bupivacaine and ropivacaine, mepivacaine, which has a similar pharmacological effect to lidocaine, is the safest among clinically used local anesthetics.
Procaine is a DNA-demethylating agent with growth-inhibitory effects in human cancer cells.[Pubmed:12941824]
Cancer Res. 2003 Aug 15;63(16):4984-9.
Methylation-associated silencing of tumor suppressor genes is recognized as being a molecular hallmark of human cancer. Unlike genetic alterations, changes in DNA methylation are potentially reversible. This possibility has attracted considerable attention from a therapeutics standpoint. Nucleoside-analogue inhibitors of DNA methyltransferases, such as 5-aza-2'-deoxycytidine, are able to demethylate DNA and restore silenced gene expression. Unfortunately, the clinical utility of these compounds has not yet been fully realized, mainly because of their side effects. A few non-nucleoside inhibitors of DNA methyltransferases have been reported, including the anti-arrhythmia drug procainamide. Following this need to find new demethylating agents, we have tested the potential use of Procaine, an anesthetic drug related to procainamide. Using the MCF-7 breast cancer cell line, we have found that Procaine is a DNA-demethylating agent that produces a 40% reduction in 5-methylcytosine DNA content as determined by high-performance capillary electrophoresis or total DNA enzyme digestion. Procaine can also demethylate densely hypermethylated CpG islands, such as those located in the promoter region of the RAR beta 2 gene, restoring gene expression of epigenetically silenced genes. This property may be explained by our finding that Procaine binds to CpG-enriched DNA. Finally, Procaine also has growth-inhibitory effects in these cancer cells, causing mitotic arrest. Thus, Procaine is a promising candidate agent for future cancer therapies based on epigenetics.
Procaine and procainamide inhibit the Wnt canonical pathway by promoter demethylation of WIF-1 in lung cancer cells.[Pubmed:19885602]
Oncol Rep. 2009 Dec;22(6):1479-84.
Secreted Wingless type (Wnt) ligands have previously been shown to be involved in tumor developmental processes and oncogenesis. Aberrant promoter methylation of Wnt inhibitory factor-1 (WIF-1) is a fundamental mechanism of epigenetic silencing in human cancers. Procaine, a local anesthetic drug, and procainamide, a drug for the treatment of cardiac arrhythmias, have been reported as inhibitors of DNA methylation, causing demethylation and reactivation of methylation-silenced genes such as RARbeta and GSTP1. The promoter demethylation of WIF-1 has not previously been reported on. We demonstrated previously that WIF-1 is silenced due to promoter hypermethylation in lung cancer cell lines. In this study, we demonstrate promoter demethylation of WIF-1; restoration of WIF-1 expression, and underexpression of cytosolic beta-catenin protein and TCF reporter activity, after Procaine and procainamide treatment in H460 and A549 cell lines. Our results provide the first evidence that Procaine and procainamide reactivate WIF-1 in these cancer cells and downregulate the Wnt canonical pathway. These results further suggest that Procaine and procainamide may have a potential use for preventing the development of lung cancer.


