Xanthurenic acidSelectively activates group II mGlu receptors CAS# 59-00-7 |
- Vinblastine Sulfate
Catalog No.:BCN2292
CAS No.:143-67-9
- Pepstatin A
Catalog No.:BCC1218
CAS No.:26305-03-3
- Dexamethasone (DHAP)
Catalog No.:BCC1184
CAS No.:50-02-2
- Omeprazole
Catalog No.:BCC1254
CAS No.:73590-58-6
- E 64d
Catalog No.:BCC1127
CAS No.:88321-09-9
Quality Control & MSDS
Number of papers citing our products

Chemical structure

3D structure
| Cas No. | 59-00-7 | SDF | Download SDF |
| PubChem ID | 5699 | Appearance | Powder |
| Formula | C10H7NO4 | M.Wt | 205.17 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : 5 mg/mL (24.37 mM; Need ultrasonic and warming) | ||
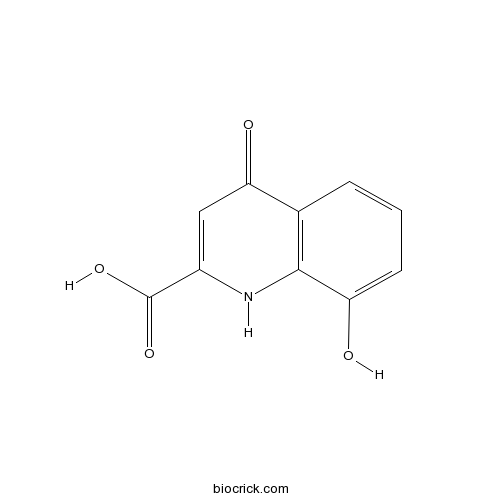
| Chemical Name | 8-hydroxy-4-oxo-1H-quinoline-2-carboxylic acid | ||
| SMILES | C1=CC2=C(C(=C1)O)NC(=CC2=O)C(=O)O | ||
| Standard InChIKey | FBZONXHGGPHHIY-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C10H7NO4/c12-7-3-1-2-5-8(13)4-6(10(14)15)11-9(5)7/h1-4,12H,(H,11,13)(H,14,15) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Shown to selectively activate group II mGlu receptors in transfected HEK293 cells at nanomolar concentrations. Attenuates cAMP formation in mouse cortical slices expressing mGlu2 and mGlu3 receptors. |

Xanthurenic acid Dilution Calculator

Xanthurenic acid Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 4.874 mL | 24.37 mL | 48.7401 mL | 97.4801 mL | 121.8502 mL |
| 5 mM | 0.9748 mL | 4.874 mL | 9.748 mL | 19.496 mL | 24.37 mL |
| 10 mM | 0.4874 mL | 2.437 mL | 4.874 mL | 9.748 mL | 12.185 mL |
| 50 mM | 0.0975 mL | 0.4874 mL | 0.9748 mL | 1.9496 mL | 2.437 mL |
| 100 mM | 0.0487 mL | 0.2437 mL | 0.4874 mL | 0.9748 mL | 1.2185 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Bestatin
Catalog No.:BCC1221
CAS No.:58970-76-6
- Cyclizine 2HCl
Catalog No.:BCC4518
CAS No.:5897-18-7
- D-Phe-Ol
Catalog No.:BCC2580
CAS No.:58917-85-4
- Z-Glu-OtBu
Catalog No.:BCC2778
CAS No.:5891-45-2
- Cassythicine
Catalog No.:BCN5802
CAS No.:5890-28-8
- Laurolitsine
Catalog No.:BCN2634
CAS No.:5890-18-6
- Monomyristin
Catalog No.:BCN8388
CAS No.:589-68-4
- Nalmefene hydrochloride
Catalog No.:BCC7857
CAS No.:58895-64-0
- Arjungenin
Catalog No.:BCN8223
CAS No.:58880-25-4
- Trichostatin A (TSA)
Catalog No.:BCC3605
CAS No.:58880-19-6
- Ophiopogonanone F
Catalog No.:BCN6409
CAS No.:588706-67-6
- Ophiopogonanone E
Catalog No.:BCN6625
CAS No.:588706-66-5
- DL-alpha-Tocopherol
Catalog No.:BCN2200
CAS No.:59-02-9
- Methotrexate
Catalog No.:BCC2301
CAS No.:59-05-2
- Ethopabate
Catalog No.:BCC8964
CAS No.:59-06-3
- 5-BrdU
Catalog No.:BCC5293
CAS No.:59-14-3
- D-Galactose
Catalog No.:BCN8528
CAS No.:59-23-4
- Folic acid
Catalog No.:BCN5375
CAS No.:59-30-3
- Mepyramine maleate
Catalog No.:BCC6740
CAS No.:59-33-6
- Sulfaquinoxaline
Catalog No.:BCC9158
CAS No.:59-40-5
- Thiamine chloride
Catalog No.:BCN8344
CAS No.:59-43-8
- Procaine
Catalog No.:BCC5210
CAS No.:59-46-1
- Oxindole
Catalog No.:BCN4050
CAS No.:59-48-3
- DL-Methionine
Catalog No.:BCC8318
CAS No.:59-51-8
Deficit, but Not Nondeficit, Schizophrenia Is Characterized by Mucosa-Associated Activation of the Tryptophan Catabolite (TRYCAT) Pathway with Highly Specific Increases in IgA Responses Directed to Picolinic, Xanthurenic, and Quinolinic Acid.[Pubmed:28181189]
Mol Neurobiol. 2018 Feb;55(2):1524-1536.
Evidence suggests that activation of the tryptophan catabolite (TRYCAT) pathway is involved in the pathophysiology of schizophrenia. However, no previous study examined whether TRYCAT pathway activation is associated with deficit schizophrenia. We measured IgA responses to TRYCATs, namely quinolinic acid, picolinic acid, kynurenic acid, Xanthurenic acid, and anthranilic acid and 3-OH-kynurenine, in 40 healthy controls and in schizophrenic patients with (n = 40) and without (n = 40) deficit, defined according to the Schedule for the Deficit Syndrome (SDS). Primary deficit schizophrenia is accompanied by an activated TRYCAT pathway as compared to controls and nondeficit schizophrenia. Participants with deficit schizophrenia show increased IgA responses to Xanthurenic acid, picolinic acid, and quinolinic acid and relatively lowered IgA responses to kynurenic and anthranilic acids, as compared to patients with nondeficit schizophrenia. Both schizophrenia subgroups show increased IgA responses to 3-OH-kynurenine as compared to controls. The IgA responses to noxious TRYCATs, namely Xanthurenic acid, picolinic acid, quinolinic acid, and 3-OH-kynurenine, but not protective TRYCATS, namely anthranilic acid and kunyrenic acid, are significantly higher in deficit schizophrenia than in controls. The negative symptoms of schizophrenia are significantly and positively associated with increased IgA responses directed against picolinic acid and inversely with anthranilic acid, whereas no significant associations between positive symptoms and IgA responses to TRYCATs were found. In conclusion, primary deficit schizophrenia is characterized by TRYCAT pathway activation and differs from nondeficit schizophrenia by a highly specific TRYCAT pattern suggesting increased excitotoxicity, cytotoxicity, and neurotoxicity, as well as inflammation and oxidative stress. The specific alterations in IgA responses to TRYCATs provide further insight for the biological delineation of deficit versus nondeficit schizophrenia.
Post-Bariatric Surgery Changes in Quinolinic and Xanthurenic Acid Concentrations Are Associated with Glucose Homeostasis.[Pubmed:27327770]
PLoS One. 2016 Jun 21;11(6):e0158051.
BACKGROUND: An increase of plasma kynurenine concentrations, potentially bioactive metabolites of tryptophan, was found in subjects with obesity, resulting from low-grade inflammation of the white adipose tissue. Bariatric surgery decreases low-grade inflammation associated with obesity and improves glucose control. OBJECTIVE: Our goal was to determine the concentrations of all kynurenine metabolites after bariatric surgery and whether they were correlated with glucose control improvement. DESIGN: Kynurenine metabolite concentrations, analysed by liquid or gas chromatography coupled with tandem mass spectrometry, circulating inflammatory markers, metabolic traits, and BMI were measured before and one year after bariatric surgery in 44 normoglycemic and 47 diabetic women with obesity. Associations between changes in kynurenine metabolites concentrations and in glucose control and metabolic traits were analysed between baseline and twelve months after surgery. RESULTS: Tryptophan and kynurenine metabolite concentrations were significantly decreased one year after bariatric surgery and were correlated with the decrease of the usCRP in both groups. Among all the kynurenine metabolites evaluated, only quinolinic acid and Xanthurenic acid were significantly associated with glucose control improvement. The one year delta of quinolinic acid concentrations was negatively associated with the delta of fasting glucose (p = 0.019) and HbA1c (p = 0.014), whereas the delta of Xanthurenic acid was positively associated with the delta of insulin sensitivity index (p = 0.0018). CONCLUSION: Bariatric surgery has induced a global down-regulation of kynurenine metabolites, associated with weight loss. Our results suggest that, since kynurenine monoxygenase diverts the kynurenine pathway toward the synthesis of Xanthurenic acid, its inhibition may also contribute to glucose homeostasis.
Urinary excretion ratio of xanthurenic acid/kynurenic acid as a functional biomarker of niacin nutritional status.[Pubmed:27429300]
Biosci Biotechnol Biochem. 2016 Nov;80(11):2208-2216.
The present study was conducted to survey functional biomarkers for evaluation of niacin nutritional status. Over 500 enzymes require niacin as a coenzyme. Of these, we chose the tryptophan degradation pathway. To create niacin-deficient animals, quinolinic acid phosphoribosyltransferase-knock out mice were used in the present study because wild type mice can synthesize nicotinamide from tryptophan. When the mice were made niacin-deficient, the urinary excretion of Xanthurenic acid (XA) was extremely low compared with control mice; however, it increased according to the recovery of niacin nutritional status. The urinary excretion of kynurenic acid (KA) was the reverse of XA. Kynurenine 3-monooxygenase, which needs NADPH, was thought to be suppressed by niacin deficiency. Thus, we calculated the urinary excretion ratio of XA:KA as a functional biomarker of niacin nutrition. The ratio increased according to recovering niacin nutritional status. Low values equate with low niacin nutritional status.
The tryptophan oxidation pathway in mosquitoes with emphasis on xanthurenic acid biosynthesis.[Pubmed:17070835]
J Insect Physiol. 2007 Mar;53(3):254-63.
Oxidation of tryptophan to kynurenine and 3-hydroxykynurenine (3-HK) is the major catabolic pathway in mosquitoes. However, 3-HK is oxidized easily under physiological conditions, resulting in the production of reactive radical species. To overcome this problem, mosquitoes have developed an efficient mechanism to prevent 3-HK from accumulating by converting this chemically reactive compound to the chemically stable Xanthurenic acid. Interestingly, 3-HK is a precursor for the production of compound eye pigments during the pupal and early adult stages; consequently, mosquitoes need to preserve and transport 3-HK for compound eye pigmentation in pupae and adults. This review summarizes the tryptophan oxidation pathway, compares and contrasts the mosquito tryptophan oxidation pathway with other model species, and discusses possible driving forces leading to the functional adaptation and evolution of enzymes involved in the mosquito tryptophan oxidation pathway.


