Trichostatin A (TSA)HDAC inhibitor CAS# 58880-19-6 |
- CUDC-101
Catalog No.:BCC2149
CAS No.:1012054-59-9
- TMP269
Catalog No.:BCC3993
CAS No.:1314890-29-3
- Vorinostat (SAHA, MK0683)
Catalog No.:BCC2145
CAS No.:149647-78-9
- LAQ824 (NVP-LAQ824,Dacinostat)
Catalog No.:BCC2160
CAS No.:404951-53-7
- JNJ-26481585
Catalog No.:BCC2147
CAS No.:875320-29-9
- Pracinostat (SB939)
Catalog No.:BCC2152
CAS No.:929016-96-6
Quality Control & MSDS
Number of papers citing our products

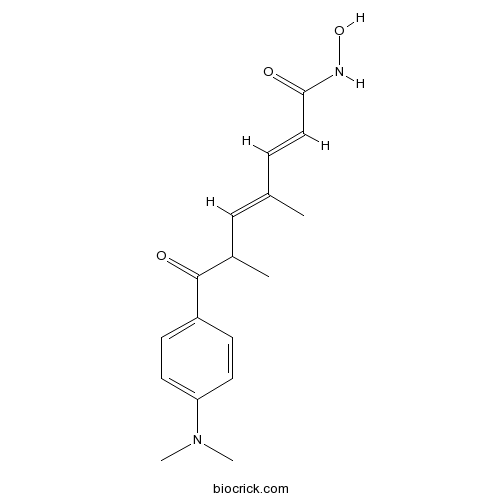
Chemical structure
3D structure
| Cas No. | 58880-19-6 | SDF | Download SDF |
| PubChem ID | 6376322 | Appearance | Powder |
| Formula | C17H22N2O3 | M.Wt | 302.37 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | TSA | ||
| Solubility | DMSO : 25 mg/mL (82.68 mM; Need ultrasonic) | ||
| Chemical Name | (2E,4E)-7-[4-(dimethylamino)phenyl]-N-hydroxy-4,6-dimethyl-7-oxohepta-2,4-dienamide | ||
| SMILES | CC(C=C(C)C=CC(=O)NO)C(=O)C1=CC=C(C=C1)N(C)C | ||
| Standard InChIKey | RTKIYFITIVXBLE-WKWSCTOISA-N | ||
| Standard InChI | InChI=1S/C17H22N2O3/c1-12(5-10-16(20)18-22)11-13(2)17(21)14-6-8-15(9-7-14)19(3)4/h5-11,13,22H,1-4H3,(H,18,20)/b10-5+,12-11+ | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Selective and potent inhibitor of histone deacetylase (Ki = 3.4 nM). Active in vivo. Potential anti-cancer agent. Induces accelerated dedifferentiation of primordial germ cells (PGCs) into embryonic germ (EG) cells. Activates autophagy. |

Trichostatin A (TSA) Dilution Calculator

Trichostatin A (TSA) Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.3072 mL | 16.536 mL | 33.0721 mL | 66.1441 mL | 82.6802 mL |
| 5 mM | 0.6614 mL | 3.3072 mL | 6.6144 mL | 13.2288 mL | 16.536 mL |
| 10 mM | 0.3307 mL | 1.6536 mL | 3.3072 mL | 6.6144 mL | 8.268 mL |
| 50 mM | 0.0661 mL | 0.3307 mL | 0.6614 mL | 1.3229 mL | 1.6536 mL |
| 100 mM | 0.0331 mL | 0.1654 mL | 0.3307 mL | 0.6614 mL | 0.8268 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Trichostatin A (TSA) is a potent inhibitor of histone deacetylase (HDAC) as well as an antifungal antibiotic with cytostatic and differentiating properties that noncompetivively and reversibly inhibits HDAC, at low nanomolar concentrations, in both cultured mammalian cells and fractionated cell nuclear extracts. It is capable of arresting cells in G1 and G2 phases of the cell cycle, inducing differentiation and reverting the transformed morphology of cells in culture. According to a study investigating the effect of TSA in human breast cancer cell lines, TSA inhibited proliferation of breast carcinoma cell lines (IC50 124.4 ± 120.4 nM), comparing to all cell lines (IC50 2.4 ± 0.5 nM), and resulted in pronounced histone H4 hyperacetylation.
Reference
David M. Vigushin, Simak Ali, Paul E. Pace, Nina Mirsaidi, Kazuhiro Ito, Ian Adcock, and R. Charles Coombes. Trichostatin A is a histone deacetylase inhibitor with potent antitumot activity against breast cancer in vivo. Clin Cancer Res 2001;7:971-976
- Ophiopogonanone F
Catalog No.:BCN6409
CAS No.:588706-67-6
- Ophiopogonanone E
Catalog No.:BCN6625
CAS No.:588706-66-5
- 9-Oxonerolidol
Catalog No.:BCN5801
CAS No.:58865-88-6
- Secoxyloganin
Catalog No.:BCN5800
CAS No.:58822-47-2
- [Leu5]-Enkephalin
Catalog No.:BCC5831
CAS No.:58822-25-6
- SB 297006
Catalog No.:BCC6129
CAS No.:58816-69-6
- Toosendanin
Catalog No.:BCN1007
CAS No.:58812-37-6
- Benzalazine
Catalog No.:BCC8843
CAS No.:588-68-1
- KU 55933
Catalog No.:BCC2475
CAS No.:587871-26-9
- ABT 724 trihydrochloride
Catalog No.:BCC7293
CAS No.:587870-77-7
- C7280948
Catalog No.:BCC6443
CAS No.:587850-67-7
- ZCL278
Catalog No.:BCC3665
CAS No.:587841-73-4
- Arjungenin
Catalog No.:BCN8223
CAS No.:58880-25-4
- Nalmefene hydrochloride
Catalog No.:BCC7857
CAS No.:58895-64-0
- Monomyristin
Catalog No.:BCN8388
CAS No.:589-68-4
- Laurolitsine
Catalog No.:BCN2634
CAS No.:5890-18-6
- Cassythicine
Catalog No.:BCN5802
CAS No.:5890-28-8
- Z-Glu-OtBu
Catalog No.:BCC2778
CAS No.:5891-45-2
- D-Phe-Ol
Catalog No.:BCC2580
CAS No.:58917-85-4
- Cyclizine 2HCl
Catalog No.:BCC4518
CAS No.:5897-18-7
- Bestatin
Catalog No.:BCC1221
CAS No.:58970-76-6
- Xanthurenic acid
Catalog No.:BCC7866
CAS No.:59-00-7
- DL-alpha-Tocopherol
Catalog No.:BCN2200
CAS No.:59-02-9
- Methotrexate
Catalog No.:BCC2301
CAS No.:59-05-2
Trichostatin A (TSA) facilitates formation of partner preference in male prairie voles (Microtus ochrogaster).[Pubmed:27074037]
Horm Behav. 2016 May;81:68-73.
In the socially monogamous prairie voles (Microtus ochrogaster), the development of a social bonding is indicated by the formation of partner preference, which involves a variety of environmental and neurochemical factors and brain structures. In a most recent study in female prairie voles, we found that treatment with the histone deacetylase inhibitor Trichostatin A (TSA) facilitates the formation of partner preference through up-regulation of oxytocin receptor (OTR) and vasopressin V1a receptor (V1aR) genes expression in the nucleus accumbens (NAcc). In the present study, we tested the hypothesis that TSA treatment also facilitates partner preference formation and alters OTR and V1aR genes expression in the NAcc in male prairie voles. We thus observed that central injection of TSA dose-dependently promoted the formation of partner preference in the absence of mating in male prairie voles. Interestingly, TSA treatment up-regulated OTR, but not V1aR, gene expression in the NAcc similarly as they were affected by mating - an essential process for naturally occurring partner preference. These data, together with others, not only indicate the involvement of epigenetic events but also the potential role of NAcc oxytocin in the regulation of partner preference in both male and female prairie voles.
Enhancement of inflammatory protein expression and nuclear factor Kappab (NF-Kappab) activity by trichostatin A (TSA) in OP9 preadipocytes.[Pubmed:23555753]
PLoS One. 2013;8(3):e59702.
The production of inflammatory proteins such as interleukin-6 (IL-6) by preadipocytes and mature adipocytes is closely associated with the impairment of systemic glucose homeostasis. However, precisely how the production is regulated and the roles of histone deacetylases (HDACs) remain largely unknown. The aim of this study was to establish whether HDAC inhibitors affect the expression of inflammatory proteins in pre/mature adipocytes, and, if so, to determine the mechanism involved. Trichostatin A (TSA), an HDAC inhibitor, enhanced lipopolysaccharide (LPS)-induced production of IL-6 in OP9 preadipocytes but not the mature adipocytes. Moreover, TSA also enhanced palmitic acid-induced IL-6 production and the expression of inflammatory genes induced by LPS in preadipocytes. Although TSA did not affect TLR4 mRNA expression or the activation of MAPKs, a reporter gene assay revealed that the LPS-induced increase in nuclear factor kappaB (NF-kappaB) activity was enhanced by TSA. Moreover, TSA increased the level of NF-kappaB p65 acetylation at lysine 310 and duration of its translocation into the nucleus, which leads to enhancement of NF-kappaB activity and subsequently expression of inflammatory genes. These findings shed new light on the regulatory roles of HDACs in preadipocytes in the production of inflammatory proteins.
Different effects of histone deacetylase inhibitors nicotinamide and trichostatin A (TSA) in C17.2 neural stem cells.[Pubmed:22407380]
J Neural Transm (Vienna). 2012 Nov;119(11):1307-15.
Histone deacetylase inhibitors are involved in proliferation, apoptosis, cell cycle, mRNA transcription, and protein expression in various cells. However, the molecular mechanism underlying such functions is still not fully clear. In this study, we used C17.2 neural stem cell (NSC) line as a model to evaluate the effects of nicotinamide and Trichostatin A (TSA) on cell characteristics. Results show that nicotinamide and TSA greatly inhibit cell growth, lead to cell morphology changes, and effectively induce cell apoptosis in a dose-dependent manner. Western blot analyses confirmed that nicotinamide significantly decreases the expression of bcl-2 and p38. Further insight into the molecular mechanisms shows the suppression of phosphorylation in eukaryotic initiation factor 4E-binding protein 1 (4EBP1) by nicotinamide, whereas, an increased expression of bcl-2 and p38 and phosphorylation of 4EBP1 by TSA. However, both nicotinamide and TSA significantly increase the expression of cytochrome c (cyt c). These results strongly suggest that bcl-2, p38, cyt c, and p-4EBP1 could suppress proliferation and induce apoptosis of C17.2 NSCs mediated by histone deacetylase inhibitors, nicotinamide and TSA, involving different molecular mechanisms.
Prevention of Pulmonary Fibrosis via Trichostatin A (TSA) in Bleomycin Induced Rats.[Pubmed:25363222]
Sarcoidosis Vasc Diffuse Lung Dis. 2014 Oct 20;31(3):219-26.
PURPOSE: To investigate the effects of non selective histone deacetylase inhibitors Trichostatin A (TSA)on bleomycin-induced pulmonary fibrosis. To investigate the effects of non selective histone deacetylase inhibitors Trichostatin A ( TSA ) on HDAC2, p-SMAD2, HDAC2 mRNA, SMAD2mRNA in pulmonary fibrosis rats and investigate impossible mechanism. METHODS: 46 SPF level male SD rats were randomly divided into four groups: ten for normal control group, fourteen for model control group I, twelve for model control group II and ten for treatment group. Rat pulmonary fibrosis was induced by bleomycin(5mg/kg) via single intratracheal perfusion in the two model control groups and treatment group. Normal control mice were instilled with a corresponding volume of 0.9% saline intratracheally. Treatment group was treated by the dilution of TSA 2mg/kg DMSO 60ul and0.9% saline 1.2ml intraperitoneal injection from the next day ,once a day for three days. Model control group II was treated by the dilution of DMSO 60ul and0.9% saline 1.2ml intraperitoneal injection from the next day once a day for three days. Model control group I and normal control group were treated by 0.9% saline 1.2ml intraperitoneal injection from the next day once a day for three days. All the animals were sacrificed on the 21 day after modeling. The pathological changes were observed by hematoxylin and eosin(HE)stain and masson trichrome stain. The expression of HDAC2 mRNA,SMAD2 mRNA were measured by real-time PCR. The protein level of HDAC2 and p-SMAD2 in serum was measured by Western blot. RESULTS: The pulmonary fibrosis in treatment group were significantly alleviated compared to the two model control groups (P<0.05). Real-time PCR showed that the treatment group had lower expression of lung tissue HDAC2 mRNA than the two model control groups and normal control group (P<0.05). The expression of lung tissue SMAD2 mRNA increased in the two model control groups and treatment group (P<0.05),but there were no significant differences among the three groups(P>0.05). Western blot indicated that the protein level of HDAC2 and p-SMAD2 in serum increased in the two model control groups compared with normal control group(P<0.05).But treatment group had lower protein level of HDAC2 (P<0.05) and no significant difference in the protein level of p-SMAD2 compared to the two model control groups (P>0.05). CONCLUSION: Non selective histone deacetylase inhibitors of Trichostatin A (TSA) can reduce the bleomycin induced pulmonary fibrosis in rats. TSA attenuates pulmonary fibrosis and it can inhibit HDAC2 expression at the gene and protein level. Bleomycin induced fibrosis has the relationship with p-SMAD2 in gene and protein levels, but TSA inhibit bleomycin-induced lung fibrosis effect with no relation with SMAD2 phosphorylation pathways.
Reprogramming primordial germ cells into pluripotent stem cells.[Pubmed:18953407]
PLoS One. 2008;3(10):e3531.
BACKGROUND: Specification of primordial germ cells (PGCs) results in the conversion of pluripotent epiblast cells into monopotent germ cell lineage. Blimp1/Prmt5 complex plays a critical role in the specification and maintenance of the early germ cell lineage. However, PGCs can be induced to dedifferentiate back to a pluripotent state as embryonic germ (EG) cells when exposed to exogenous signaling molecules, FGF-2, LIF and SCF. METHODOLOGY AND PRINCIPAL FINDINGS: Here we show that Trichostatin A (TSA), an inhibitor of histone deacetylases, is a highly potent agent that can replace FGF-2 to induce dedifferentiation of PGCs into EG cells. A key early event during dedifferentiation of PGCs in response to FGF-2 or TSA is the down-regulation of Blimp1, which reverses and apparently relieves the cell fate restriction imposed by it. Notably, the targets of Blimp1, which include c-Myc and Klf-4, which represent two of the key factors known to promote reprogramming of somatic cells to pluripotent state, are up-regulated. We also found early activation of the LIF/Stat-3 signaling pathway with the translocation of Stat-3 into the nucleus. By contrast, while Prmt5 is retained in EG cells, it translocates from the nucleus to the cytoplasm where it probably has an independent role in regulating pluripotency. CONCLUSIONS/SIGNIFICANCE: We propose that dedifferentiation of PGCs into EG cells may provide significant mechanistic insights on early events associated with reprogramming of committed cells to a pluripotent state.
Trichostatin A is a histone deacetylase inhibitor with potent antitumor activity against breast cancer in vivo.[Pubmed:11309348]
Clin Cancer Res. 2001 Apr;7(4):971-6.
PURPOSE: Trichostatin A (TSA), an antifungal antibiotic with cytostatic and differentiating properties in mammalian cell culture, is a potent and specific inhibitor of histone deacetylase (HDAC) activity. The purpose of this study was to evaluate the antiproliferative and HDAC inhibitory activity of TSA in vitro in human breast cancer cell lines and to assess its antitumor efficacy and toxicity in vivo in a carcinogen-induced rat mammary cancer model. EXPERIMENTAL DESIGN AND RESULTS: TSA inhibited proliferation of eight breast carcinoma cell lines with mean +/- SD IC(50) of 124.4 +/- 120.4 nM (range, 26.4-308.1 nM). HDAC inhibitory activity of TSA was similar in all cell lines with mean +/- SD IC(50) of 2.4 +/- 0.5 nM (range, 1.5-2.9 nM), and TSA treatment resulted in pronounced histone H4 hyperacetylation. In randomized controlled efficacy studies using the N-methyl-N-nitrosourea carcinogen-induced rat mammary carcinoma model, TSA had pronounced antitumor activity in vivo when administered to 16 animals at a dose of 500 microg/kg by s.c. injection daily for 4 weeks compared with 14 control animals. Furthermore, TSA did not cause any measurable toxicity in doses of up to 5 mg/kg by s.c. injection. Forty-one tumors from 26 animals were examined by histology. Six tumors from 3 rats treated with TSA and 14 tumors from 9 control animals were adenocarcinomas. In contrast, 19 tumors from 12 TSA-treated rats had a benign phenotype, either fibroadenoma or tubular adenoma, suggesting that the antitumor activity of TSA may be attributable to induction of differentiation. Two control rats each had tumors with benign histology. CONCLUSIONS: The present studies confirm the potent dose-dependent antitumor activity of TSA against breast cancer in vitro and in vivo, strongly supporting HDAC as a molecular target for anticancer therapy in breast cancer.
Inhibition of mitogenesis in Balb/c-3T3 cells by Trichostatin A. Multiple alterations in the induction and activation of cyclin-cyclin-dependent kinase complexes.[Pubmed:10945992]
J Biol Chem. 2000 Oct 27;275(43):33981-7.
Trichostatin A (TSA), a global repressor of histone deacetylase activity, inhibits the proliferation of a number of cell types. However, the identification of the mechanisms underlying TSA-mediated growth arrests has remained elusive. In order to resolve in more detail the cellular process modulated during the growth inhibition induced by TSA, we studied the effect of the drug on G(0)/G(1) traverse in mitogen-stimulated quiescent Balb/c-3T3 cells. Cyclin D1 and retinoblastoma proteins were induced following the mitogenic stimulation of both control and TSA-treated cells, and cyclin D1 formed complexes with CDK4 under both conditions. However, cyclin D1-associated kinase was not increased in growth-arrested cells. The lack of cyclin D-associated kinase was paralleled by an accumulation of RB in a hypophosphorylated form, as would be expected. In contrast, p130 became partially phosphorylated, accompanied by a marked increase in p130-dependent E2F DNA binding activity and a partial release of free E2F-4. Despite the presence of E2F complexes not bound to pocket proteins, late G(1) E2F-dependent gene expression was not observed. The lack of cyclin D1-associated kinase in TSA-treated cultures was potentially due to high levels of the cyclin-dependent inhibitor p27(kip1). However, the modulation of p27(kip1) levels by the deacetylase inhibitor cannot be responsible for the induction of the cell cycle arrest, since the growth of murine embryo fibroblasts deficient in both p27(kip1) and p21(cip1) was also inhibited by TSA. These data support a model in which TSA inhibits very early cell cycle traverse, which, in turn, leads to a decrease in cyclin D1-associated kinase activation and a repression of late cell cycle-dependent events. Alterations in early G(0)/G(1) gene expression accompany the TSA-mediated growth arrest.
Potent and specific inhibition of mammalian histone deacetylase both in vivo and in vitro by trichostatin A.[Pubmed:2211619]
J Biol Chem. 1990 Oct 5;265(28):17174-9.
(R)-Trichostatin A (TSA) is a Streptomyces product which causes the induction of Friend cell differentiation and specific inhibition of the cell cycle of normal rat fibroblasts in the G1 and G2 phases at the very low concentrations. We found that TSA caused an accumulation of acetylated histone species in a variety of mammalian cell lines. Pulse-labeling experiments indicated that TSA markedly prolonged the in vivo half-life of the labile acetyl groups on histones in mouse mammary gland tumor cells, FM3A. The partially purified histone deacetylase from wild-type FM3A cells was effectively inhibited by TSA in a noncompetitive manner with Ki = 3.4 nM. A newly isolated mutant cell line of FM3A resistant to TSA did not show the accumulation of the acetylated histones in the presence of a higher concentration of TSA. The histone deacetylase preparation from the mutant showed decreased sensitivity to TSA (Ki = 31 nM, noncompetitive). These results clearly indicate that TSA is a potent and specific inhibitor of the histone deacetylase and that the in vivo effect of TSA on cell proliferation and differentiation can be attributed to the inhibition of the enzyme.


