PyBrOPCAS# 132705-51-2 |
- BS-181
Catalog No.:BCC1439
CAS No.:1092443-52-1
- CDK inhibitor II
Catalog No.:BCC1464
CAS No.:1269815-17-9
- CGP60474
Catalog No.:BCC1474
CAS No.:164658-13-3
- SNS-032 (BMS-387032)
Catalog No.:BCC1152
CAS No.:345627-80-7
- Dinaciclib (SCH727965)
Catalog No.:BCC3765
CAS No.:779353-01-4
- AT7519
Catalog No.:BCC2541
CAS No.:844442-38-2
Quality Control & MSDS
Number of papers citing our products

Chemical structure

3D structure
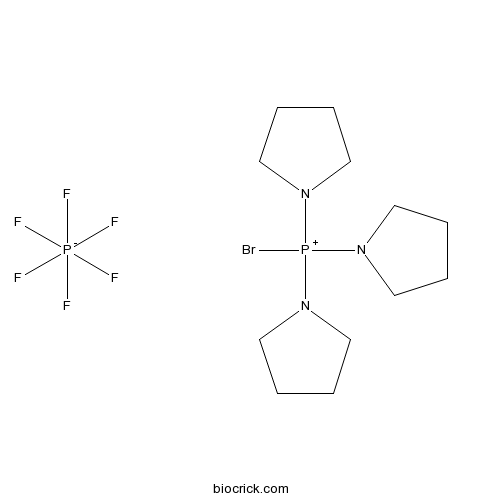
| Cas No. | 132705-51-2 | SDF | Download SDF |
| PubChem ID | 2733179 | Appearance | Powder |
| Formula | C12H24N3P2BrF6 | M.Wt | 466.2 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in water or 1% acetic acid | ||
| Chemical Name | bromo(tripyrrolidin-1-yl)phosphanium;hexafluorophosphate | ||
| SMILES | C1CCN(C1)[P+](N2CCCC2)(N3CCCC3)Br.F[P-](F)(F)(F)(F)F | ||
| Standard InChIKey | CYKRMWNZYOIJCH-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C12H24BrN3P.F6P/c13-17(14-7-1-2-8-14,15-9-3-4-10-15)16-11-5-6-12-16;1-7(2,3,4,5)6/h1-12H2;/q+1;-1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

PyBrOP Dilution Calculator

PyBrOP Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.145 mL | 10.725 mL | 21.45 mL | 42.9 mL | 53.6251 mL |
| 5 mM | 0.429 mL | 2.145 mL | 4.29 mL | 8.58 mL | 10.725 mL |
| 10 mM | 0.2145 mL | 1.0725 mL | 2.145 mL | 4.29 mL | 5.3625 mL |
| 50 mM | 0.0429 mL | 0.2145 mL | 0.429 mL | 0.858 mL | 1.0725 mL |
| 100 mM | 0.0215 mL | 0.1073 mL | 0.2145 mL | 0.429 mL | 0.5363 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
PyBrOP
- [Leu31,Pro34]-Neuropeptide Y (human, rat)
Catalog No.:BCC5722
CAS No.:132699-73-1
- Fmoc-Tle-OH
Catalog No.:BCC2657
CAS No.:132684-60-7
- Senecionine N-oxide
Catalog No.:BCN2130
CAS No.:13268-67-2
- Otophylloside O
Catalog No.:BCN7337
CAS No.:1326583-08-7
- CCMQ
Catalog No.:BCC6984
CAS No.:132623-44-0
- Bruceanic acid C
Catalog No.:BCN7999
CAS No.:132587-60-1
- 15-Demethylplumieride
Catalog No.:BCN6176
CAS No.:132586-69-7
- Olanzapine
Catalog No.:BCC5042
CAS No.:132539-06-1
- (Z)-Pugnac
Catalog No.:BCC5333
CAS No.:132489-69-1
- 2-TEDC
Catalog No.:BCC6735
CAS No.:132465-10-2
- Alosetron-d3 Hydrochloride
Catalog No.:BCC1345
CAS No.:1189919-71-8
- Neohesperidin
Catalog No.:BCN5915
CAS No.:13241-33-3
- CP 96345
Catalog No.:BCC7509
CAS No.:132746-60-2
- Blonanserin
Catalog No.:BCC3740
CAS No.:132810-10-7
- YS-49
Catalog No.:BCC2067
CAS No.:132836-42-1
- Terchebulin
Catalog No.:BCN3264
CAS No.:132854-40-1
- PD 81723
Catalog No.:BCC7032
CAS No.:132861-87-1
- Lercanidipine hydrochloride
Catalog No.:BCC5238
CAS No.:132866-11-6
- HOE-S 785026
Catalog No.:BCC1633
CAS No.:132869-83-1
- Bis(4-hydroxy-3,5-dimethylphenyl) sulfone
Catalog No.:BCC8884
CAS No.:13288-70-5
- Ramosetron Hydrochloride
Catalog No.:BCC5272
CAS No.:132907-72-3
- 9-Hydroxy-13E-labden-15-oic acid
Catalog No.:BCN6177
CAS No.:132915-47-0
- Rifampin
Catalog No.:BCC4839
CAS No.:13292-46-1
- Macrocarpal A
Catalog No.:BCN6178
CAS No.:132951-90-7
Efficient Pd-catalyzed coupling of tautomerizable heterocycles with terminal alkynes via C-OH bond activation using PyBrOP.[Pubmed:20411949]
Org Lett. 2010 May 21;12(10):2286-9.
The direct alkynylation of tautomerizable heterocylcles is described via a two-step process involving in situ C-OH activation with bromotripyrrolidinophosphonium hexafluorophosphate (PyBrOP) followed by Sonogashira coupling with a wide range of alkyl or aryl terminal alkynes using a copper-free system employing PdCl(2)(CH(3)CN)(2) and 2-(dicyclohexylphosphino)biphenyl.
Ni-catalyzed construction of C-P bonds from electron-deficient phenols via the in situ aryl C-O activation by PyBroP.[Pubmed:22573216]
Chem Commun (Camb). 2012 Jun 14;48(47):5868-70.
The C-P bond forming reaction using electron-deficient phenol substrates was considerably challenging. Herein, we present a new protocol that allows for one-pot construction of C-P bonds via the cross-coupling of phenols and phosphine oxide or phosphite in the presence of a nickel catalyst.
Nickel-catalyzed cross-coupling of phenols and arylboronic acids through an in situ phenol activation mediated by PyBroP.[Pubmed:21360600]
Chemistry. 2011 Mar 28;17(14):4038-42.
A new method for the Suzuki-Miyaura cross-coupling of phenols and arylboronic acids through in situ phenol activation mediated by PyBrOP is presented. The reaction proceeds efficiently by using cost-effective, markedly stable [NiCl(2)(dppp)] (dppp=1,3-bis(diphenylphosphino)propane) as the catalyst in only 5 mol % loading, as well as in the absence of extra ligands. The method exhibits broad applicability and high efficiency towards a wide range of both phenols and boronic acids, including activated, nonactivated, deactivated, and heteroaromatic coupling partners. In addition, various functional groups, such as ether, amino, cyano, ester, and ketone groups, are compatible with this transformation. Notably, arylboronic acids containing an unprotected NH(2) group and 2-heterocyclic boronic acids, which are generally problematic for coupling under conventional conditions, are also viable substrates, although moderate yields were obtained for sterically hindered substrates. Consequently, the in situ cross-coupling methodology coupled with the use of an inexpensive and stable nickel catalyst provides a rapid and efficient pathway for the assembly of biaryls and heterobiaryls with structural diversity from readily available phenol compounds.
PdCl2(dppf)-catalyzed in situ coupling of 2-hydroxypyridines with aryl boronic acids mediated by PyBroP and the one-pot chemo- and regioselective construction of two distinct aryl-aryl bonds.[Pubmed:22045165]
Chem Commun (Camb). 2011 Dec 28;47(48):12840-2.
We present a PdCl(2)(dppf)-catalyzed synthesis of 2-arylated pyridine derivatives via the in situ coupling of 2-OH pyridines and boronic acids mediated by PyBrOP. In addition, the highly chemo- and regioselective construction of two different aryl-aryl bonds via a one-pot operation has also been demonstrated by the orthogonal use of this method with the Ni-catalyzed Suzuki-Miyaura coupling of phenols.


