BlonanserinAtypical antipsychotic CAS# 132810-10-7 |
- CX-5461
Catalog No.:BCC3700
CAS No.:1138549-36-6
- Gemcitabine HCl
Catalog No.:BCC1076
CAS No.:122111-03-9
- Fludarabine
Catalog No.:BCC2518
CAS No.:21679-14-1
- Carboplatin
Catalog No.:BCC1170
CAS No.:41575-94-4
- Epirubicin HCl
Catalog No.:BCC1192
CAS No.:56390-09-1
- Fludarabine Phosphate (Fludara)
Catalog No.:BCC3681
CAS No.:75607-67-9
Quality Control & MSDS
Number of papers citing our products

Chemical structure

3D structure
| Cas No. | 132810-10-7 | SDF | Download SDF |
| PubChem ID | 125564 | Appearance | Powder |
| Formula | C23H30FN3 | M.Wt | 367.5 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : 14.29 mg/mL (38.88 mM; Need ultrasonic) | ||
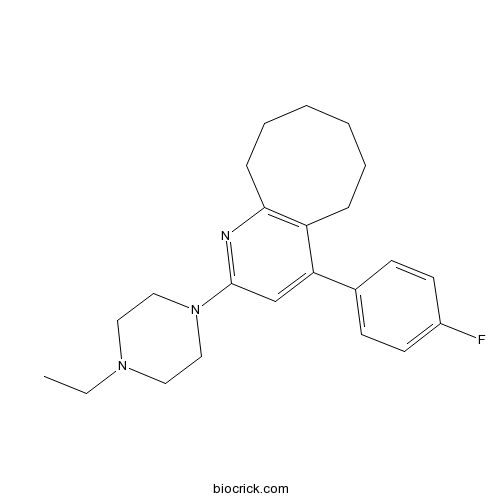
| Chemical Name | 2-(4-ethylpiperazin-1-yl)-4-(4-fluorophenyl)-5,6,7,8,9,10-hexahydrocycloocta[b]pyridine | ||
| SMILES | CCN1CCN(CC1)C2=NC3=C(CCCCCC3)C(=C2)C4=CC=C(C=C4)F | ||
| Standard InChIKey | XVGOZDAJGBALKS-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C23H30FN3/c1-2-26-13-15-27(16-14-26)23-17-21(18-9-11-19(24)12-10-18)20-7-5-3-4-6-8-22(20)25-23/h9-12,17H,2-8,13-16H2,1H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Blonanserin Dilution Calculator

Blonanserin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.7211 mL | 13.6054 mL | 27.2109 mL | 54.4218 mL | 68.0272 mL |
| 5 mM | 0.5442 mL | 2.7211 mL | 5.4422 mL | 10.8844 mL | 13.6054 mL |
| 10 mM | 0.2721 mL | 1.3605 mL | 2.7211 mL | 5.4422 mL | 6.8027 mL |
| 50 mM | 0.0544 mL | 0.2721 mL | 0.5442 mL | 1.0884 mL | 1.3605 mL |
| 100 mM | 0.0272 mL | 0.1361 mL | 0.2721 mL | 0.5442 mL | 0.6803 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Blonanserin (Lonasen) is a relatively new atypical antipsychotic commercialized by Dainippon Sumitomo Pharma in Japan and Korea for the treatment of schizophrenia. Relative to many other antipsychotics, blonanserin has an improved tolerability profile, lacking side effects such as extrapyramidal symptoms, excessive sedation, or hypotension. As with many second-generation (atypical) antipsychotics it is significantly more efficacious in the treatment of the negative symptoms of schizophrenia compared to first-generation (typical) antipsychotics such as haloperidol.
- CP 96345
Catalog No.:BCC7509
CAS No.:132746-60-2
- PyBrOP
Catalog No.:BCC2821
CAS No.:132705-51-2
- [Leu31,Pro34]-Neuropeptide Y (human, rat)
Catalog No.:BCC5722
CAS No.:132699-73-1
- Fmoc-Tle-OH
Catalog No.:BCC2657
CAS No.:132684-60-7
- Senecionine N-oxide
Catalog No.:BCN2130
CAS No.:13268-67-2
- Otophylloside O
Catalog No.:BCN7337
CAS No.:1326583-08-7
- CCMQ
Catalog No.:BCC6984
CAS No.:132623-44-0
- Bruceanic acid C
Catalog No.:BCN7999
CAS No.:132587-60-1
- 15-Demethylplumieride
Catalog No.:BCN6176
CAS No.:132586-69-7
- Olanzapine
Catalog No.:BCC5042
CAS No.:132539-06-1
- (Z)-Pugnac
Catalog No.:BCC5333
CAS No.:132489-69-1
- 2-TEDC
Catalog No.:BCC6735
CAS No.:132465-10-2
- YS-49
Catalog No.:BCC2067
CAS No.:132836-42-1
- Terchebulin
Catalog No.:BCN3264
CAS No.:132854-40-1
- PD 81723
Catalog No.:BCC7032
CAS No.:132861-87-1
- Lercanidipine hydrochloride
Catalog No.:BCC5238
CAS No.:132866-11-6
- HOE-S 785026
Catalog No.:BCC1633
CAS No.:132869-83-1
- Bis(4-hydroxy-3,5-dimethylphenyl) sulfone
Catalog No.:BCC8884
CAS No.:13288-70-5
- Ramosetron Hydrochloride
Catalog No.:BCC5272
CAS No.:132907-72-3
- 9-Hydroxy-13E-labden-15-oic acid
Catalog No.:BCN6177
CAS No.:132915-47-0
- Rifampin
Catalog No.:BCC4839
CAS No.:13292-46-1
- Macrocarpal A
Catalog No.:BCN6178
CAS No.:132951-90-7
- Mequindox
Catalog No.:BCC9021
CAS No.:13297-17-1
- Fmoc-Lys(Fmoc)-OPfp
Catalog No.:BCC3522
CAS No.:132990-14-8
Blonanserin treatment in patients with methamphetamine-induced psychosis comorbid with intellectual disabilities.[Pubmed:28008256]
Neuropsychiatr Dis Treat. 2016 Dec 14;12:3195-3198.
OBJECTIVE: Methamphetamine (MA) use has recently been associated with high levels of psychiatric hospitalization and serious social dysfunction. MA use causes frequent psychotic symptoms, which can be treated with antipsychotics. However, people with intellectual disabilities (ID) are vulnerable to adverse effects resulting from treatment with antipsychotic medications. METHOD: We report two cases of MA-induced psychosis (MAP) in patients with ID who were treated with the antipsychotic Blonanserin. RESULTS: In both the cases presented, symptoms of psychosis were improved by switching medications from other antipsychotic drugs to Blonanserin. Despite the presence of ID in these patients, no significant adverse effects, such as sedation, were detected after treatment with Blonanserin. CONCLUSION: Blonanserin may be an effective and well-tolerated pharmacotherapeutical treatment for patients with MAP comorbid with ID. However, further work is necessary to validate this claim.
A randomized trial of aripiprazole vs blonanserin for the treatment of acute schizophrenia and related disorders.[Pubmed:27932884]
Neuropsychiatr Dis Treat. 2016 Nov 28;12:3041-3049.
OBJECTIVE: There has been no direct comparison of aripiprazole and Blonanserin for schizophrenia treatment. We conducted a 24-week, rater-masked, randomized trial of aripiprazole (6-30 mg/d) vs Blonanserin (4-24 mg/d) in schizophrenia patients who were not taking any antipsychotic medication for more than 2 weeks before enrollment (UMIN000011194). METHODS: The primary outcome measure for efficacy was improvement of Positive and Negative Syndrome Scale (PANSS) total score at week 24. Secondary outcomes were PANSS subscale scores, 21-item Hamilton Rating Scale for Depression (HAMD-21) score, response rate, discontinuation rate, and individual adverse events. RESULTS: Forty-four patients were recruited. The discontinuation rate was 86.4% in the aripiprazole group and 68.2% in the Blonanserin treatment group. There was no significant difference in mean time to discontinuation between the groups. Although both treatment groups showed significant reductions in the PANSS total score, PANSS subscale scores, and HAMD-21 scores at week 24, the magnitudes of the changes did not differ between the groups. There were no significant differences in the incidences of adverse events including somnolence, extrapyramidal symptoms, prolactin-related adverse events, and weight change between the groups. CONCLUSION: Our results suggest similar efficacy and safety profiles of aripiprazole and Blonanserin in the patients with schizophrenia. Double-blind controlled studies are needed to further explore the efficacy and safety of aripiprazole and Blonanserin in schizophrenia.
Effectiveness of blonanserin for patients with drug treatment-resistant schizophrenia and dopamine supersensitivity: A retrospective analysis.[Pubmed:27931902]
Asian J Psychiatr. 2016 Dec;24:28-32.
OBJECTIVE: Dopamine supersensitivity psychosis (DSP) is one of the key factors contributing to the development of antipsychotic treatment-resistant schizophrenia (TRS). We investigated the efficacy of Blonanserin, an atypical antipsychotic, for patients with TRS and DSP. METHODS: In this 12-month retrospective follow-up study, we investigated the cases of eight consecutive patients with unstable TRS and DSP treated with Blonanserin as an add-on therapy. We examined changes in scores for the Brief Psychiatric Rating Scale (BPRS), Clinical Global Impression-Severity of Illness (CGI-S) scale and the Global Assessment of Functioning scale (GAF) during the 12 months after the administration of Blonanserin. RESULTS: The patients' total scores on the BPRS and GAF scores were significantly improved by 3 months at the latest. Positive BPRS and CGI-S scores were also improved by 6 months at the latest. The total chlorpromazine-equivalent doses of antipsychotics were significantly reduced from 1462.3+/-499.6mg to 794.1+/-642.8mg (p=0.001) after 12 months of Blonanserin treatment, with a favorable safety and tolerability profile. CONCLUSIONS: Blonanserin may be a promising antipsychotic for the treatment of TRS and DSP.
Blonanserin - A Novel Antianxiety and Antidepressant Drug? An Experimental Study.[Pubmed:27790460]
J Clin Diagn Res. 2016 Sep;10(9):FC17-FC21.
INTRODUCTION: Many psychiatric disorders show signs and symptoms of anxiety and depression. A drug with both, effects and lesser adverse effects is always desired. Blonanserin is a novel drug with postulated effect on anxiety and depression. AIM: The study was aimed to evaluate the effect of Blonanserin on anxiety and depression in animal models. MATERIALS AND METHODS: By using elevated plus maze test and forced swimming test, the antianxiety and antidepressant effects were evaluated. Animal ethics protocols were followed strictly. Total 50 rats (10 rats per group) were used for each test. As a control drug diazepam and imipramine were used in elevated plus maze and forced swimming test respectively. Blonanserin was tested for 3 doses 0.075, 0.2 and 0.8mg. These doses were selected from previous references as well as by extrapolating human doses. RESULTS: This study showed an antianxiety effect of Blonanserin comparable to diazepam, which was statistically significant. Optimal effect was observed with 0.075mg, followed by 0.2 and 0.8mg. It also showed an antidepressant effect which was statistically significant. Optimal effect was observed at 0.2mg dose. CONCLUSION: The results showed that at a dose range of 0.075 and 0.2mg Blonanserin has potential to exert an adjuvant antianxiety and antidepressant activity in animal models. In order to extrapolate this in patient, longer clinical studies with comparable doses should be planned. The present study underlines potential of Blonanserin as a novel drug for such studies.


