FludarabineDNA synthsis inhibitor CAS# 21679-14-1 |
- CX-5461
Catalog No.:BCC3700
CAS No.:1138549-36-6
- Gemcitabine HCl
Catalog No.:BCC1076
CAS No.:122111-03-9
- Fludarabine
Catalog No.:BCC2518
CAS No.:21679-14-1
- Carboplatin
Catalog No.:BCC1170
CAS No.:41575-94-4
- Epirubicin HCl
Catalog No.:BCC1192
CAS No.:56390-09-1
- Bleomycin Sulfate
Catalog No.:BCC3694
CAS No.:9041-93-4
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 21679-14-1 | SDF | Download SDF |
| PubChem ID | 657237 | Appearance | Powder |
| Formula | C10H12FN5O4 | M.Wt | 285.23 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : 25 mg/mL (87.65 mM; Need ultrasonic) | ||
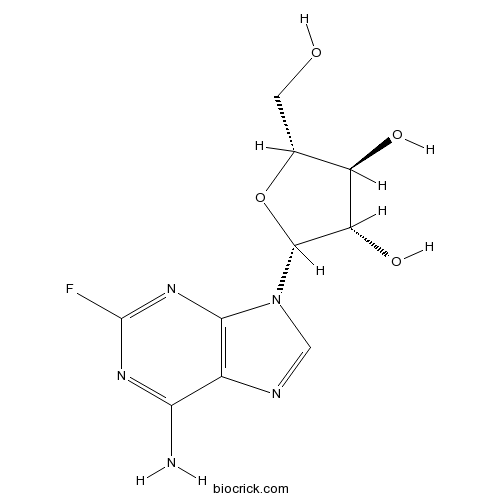
| Chemical Name | (2R,3S,4S,5R)-2-(6-amino-2-fluoropurin-9-yl)-5-(hydroxymethyl)oxolane-3,4-diol | ||
| SMILES | C1=NC2=C(N1C3C(C(C(O3)CO)O)O)N=C(N=C2N)F | ||
| Standard InChIKey | HBUBKKRHXORPQB-FJFJXFQQSA-N | ||
| Standard InChI | InChI=1S/C10H12FN5O4/c11-10-14-7(12)4-8(15-10)16(2-13-4)9-6(19)5(18)3(1-17)20-9/h2-3,5-6,9,17-19H,1H2,(H2,12,14,15)/t3-,5-,6+,9-/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Purine analog that inhibits DNA synthesis. Exhibits antiproliferative activity (IC50 = 1.54 μM in RPMI cells) and triggers apoptosis through increasing Bax and decreasing Bid, XIAP and survivin expression. Inhibits cytokine-induced activation of STAT1 and STAT1-dependent gene transcription in lymphocytes. Also displays anticancer activity against hematological malignancies in vivo. |

Fludarabine Dilution Calculator

Fludarabine Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.5059 mL | 17.5297 mL | 35.0594 mL | 70.1189 mL | 87.6486 mL |
| 5 mM | 0.7012 mL | 3.5059 mL | 7.0119 mL | 14.0238 mL | 17.5297 mL |
| 10 mM | 0.3506 mL | 1.753 mL | 3.5059 mL | 7.0119 mL | 8.7649 mL |
| 50 mM | 0.0701 mL | 0.3506 mL | 0.7012 mL | 1.4024 mL | 1.753 mL |
| 100 mM | 0.0351 mL | 0.1753 mL | 0.3506 mL | 0.7012 mL | 0.8765 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Fludarabine is a purine analog that inhibits DNA synthesis [1].
DNA synthesis is a natural creation of deoxyribonucleic acid (DNA) molecules and plays an important role in cell growth.
Fludarabine is a prodrug and is phosphorylated to the nucleoside triphosphate (F-ara-ATP) in cells to elicit biological activity. It affected a series of enzymes that required in DNA synthesis such as DNA primase, DNA polymerases, DNA ligase I, ribonucleotide reductase and 3’-5’ exonuclease activity of DNA polymerases δ and ε [1]. In human myeloma cell RPMI8226, fludarabine significantly inhibited cells growth and reduced phosphorylation of Akt. Also, it reduced XIAP and survivin, the inhibitors of apoptosis proteins (IAP) family [2]. Fludarabine can act as a cytosolic 50-nucleotidase II (cN-II) inhibitor [3].
In immunodeficient mice bearing RPMI8226 myeloma xenografts, fludarabine (40 mg/kg) slowed down the growth of tumors by about 5-fold in 25 d comparing with the control tumors [2].
References:
[1]. Gandhi V, Huang P, Plunkett W. Fludarabine inhibits DNA replication: a rationale for its use in the treatment of acute leukemias. Leuk Lymphoma, 1994, 14 Suppl 2: 3-9.
[2]. Meng H, Yang C, Ni W, et al. Antitumor activity of fludarabine against human multiple myeloma in vitro and in vivo. Eur J Haematol, 2007, 79(6): 486-493.
[3]. Cividini F, Pesi R, Chaloin L, et al. The purine analog fludarabine acts as a cytosolic 5'-nucleotidase II inhibitor. Biochem Pharmacol, 2015, 94(2): 63-68.
- Shoreic acid
Catalog No.:BCN4928
CAS No.:21671-00-1
- Bisphenol P
Catalog No.:BCC8891
CAS No.:2167-51-3
- Sophoflavescenol
Catalog No.:BCN2891
CAS No.:216450-65-6
- N-Demethylricinine
Catalog No.:BCC9098
CAS No.:21642-98-8
- Isoquerglanin
Catalog No.:BCC8189
CAS No.:143519-53-3
- 3',4',5',3,5,7,8-Heptamethoxyflavone
Catalog No.:BCN4927
CAS No.:21634-52-6
- α-Conotoxin AuIB
Catalog No.:BCC5975
CAS No.:216299-21-7
- 1alpha, 25-Dihydroxy VD2-D6
Catalog No.:BCC1299
CAS No.:216244-04-1
- Bis(2,6-diisopropylphenyl)carbodiimide
Catalog No.:BCC8879
CAS No.:2162-74-5
- β-Pompilidotoxin
Catalog No.:BCC1048
CAS No.:216064-36-7
- 1-Methyl-3-nitrophthalate
Catalog No.:BCC8468
CAS No.:21606-04-2
- 15,16-Epoxy-12S-hydroxylabda-8(17),13(16),14-triene
Catalog No.:BCN1491
CAS No.:216011-55-1
- Kulinone
Catalog No.:BCN7954
CAS No.:21688-61-9
- Lauroscholtzine
Catalog No.:BCN4929
CAS No.:2169-44-0
- Z-N-Me-Ala-OH
Catalog No.:BCC3344
CAS No.:21691-41-8
- Z-DL-Nva-OH
Catalog No.:BCC3304
CAS No.:21691-44-1
- Procurcumenol
Catalog No.:BCN3555
CAS No.:21698-40-8
- Shyobunone
Catalog No.:BCN4930
CAS No.:21698-44-2
- 15-Epi-Danshenol-A
Catalog No.:BCN3146
CAS No.:216987-13-2
- WB 4101 hydrochloride
Catalog No.:BCC6858
CAS No.:2170-58-3
- Apelin-17 (human, bovine)
Catalog No.:BCC5959
CAS No.:217082-57-0
- [Pyr1]-Apelin-13
Catalog No.:BCC7358
CAS No.:217082-60-5
- Esomeprazole Magnesium trihydrate
Catalog No.:BCC1559
CAS No.:217087-09-7
- Hugorosenone
Catalog No.:BCN3776
CAS No.:217096-49-6
The role of combined fludarabine, cyclophosphamide and rituximab chemoimmunotherapy in chronic lymphocytic leukemia: current evidence and controversies.[Pubmed:28246553]
Ther Adv Hematol. 2017 Mar;8(3):99-105.
Chemoimmunotherapy (CIT) has become a cornerstone in the treatment of patients with chronic lymphocytic leukemia (CLL). The combination of Fludarabine, cyclophosphamide and rituximab (FCR) has emerged as the standard of care for therapy of previously untreated patients with CLL who are younger than 65 years and have no significant comorbidities. In this article, we review the role of FCR in the current treatment paradigm for CLL.
Clofarabine-based chemotherapy as a bridge to transplant in the setting of refractory or relapsed acute myeloid leukemia, after at least one previous unsuccessful salvage treatment containing fludarabine: a single institution experience.[Pubmed:28220349]
Int J Hematol. 2017 Jun;105(6):769-776.
For refractory or relapsed acute myeloid leukemia patients, allogeneic hematopoietic stem cell transplantation is the only curative treatment option, but the disease must be in remission before this can be attempted. "Salvage" therapy regimens containing high-dose cytarabine plus Fludarabine or cladribine with or without anthracyclines or plus mitoxantrone and etoposide fail in 30-50% of cases. We report the outcome of 14 patients treated with a clofarabine-based treatment administered after at least one failed Fludarabine-based "salvage" attempt in a "real life" (outside a clinical trial) context. No death related to the clofarabine-based treatment was observed. Four of the 14 patients (29%) reached complete remission and one (7%) achieved a reduction of marrow blasts to fewer than 10%. Three of these five patients were successfully transplanted and have shown a long-term survival. The small number of this group of patients does not permit the identification of clinical features clearly related to a favorable outcome, but we note that all the three long-term survivals were FLT3 wild type. Clofarabine-based "salvage therapy" in patients with very poor expectancy is feasible even after a Fludarabine-based salvage attempt, albeit with success only in a small percentage of cases (3/14 = 21%).
High ten-year remission rates following rituximab, fludarabine, mitoxantrone and dexamethasone (R-FND) with interferon maintenance in indolent lymphoma: Results of a randomized Study.[Pubmed:28340281]
Br J Haematol. 2017 Apr;177(2):263-270.
We report a single-centre, randomized study evaluating the efficacy and safety of concurrent Fludarabine, mitoxantrone, dexamethasone (FND) and rituximab versus sequential FND followed by rituximab in 158 patients with advanced stage, previously untreated indolent lymphoma, enrolled between 1997 and 2002. Patients were randomized to 6-8 cycles of FND followed by 6 monthly doses of rituximab or 6 doses of rituximab given concurrently with FND. All patients who achieved at least a partial response received 12 months of interferon (IFN) maintenance. Median ages were 54 and 55 years. The two groups were comparable with the exception of a higher percentage of females (65% vs. 43%) and baseline anaemia (23% vs. 11%) in the FND followed by rituximab group. Complete response/unconfirmed complete response rates were 89% and 93%. The most frequent grade >/= 3 toxicity was neutropenia (86% vs. 96%). Neutropenic fever occurred in 21% and 16%. Late toxicity included myelodysplastic syndrome (n = 3) and acute myeloid leukaemia (n = 5). With 12.5 years of follow-up, no significant differences based on treatment schedule were observed. 10-year overall survival estimates were 76% and 73%. 10-year progression-free survival estimates were 52% and 51%. FND with concurrent or sequential rituximab, and IFN maintenance in indolent lymphoma demonstrated high response rates and robust survival.
Cost-Effectiveness Analysis of Obinutuzumab for Previously Untreated Chronic Lymphocytic Leukaemia in Portuguese Patients who are Unsuitable for Full-Dose Fludarabine-Based Therapy.[Pubmed:28342061]
Appl Health Econ Health Policy. 2017 Aug;15(4):501-512.
BACKGROUND: Chronic lymphocytic leukaemia (CLL) mostly affects patients with comorbidities and limited therapeutic options. Obinutuzumab in combination with chlorambucil (GClb) is a new therapeutic option for previously untreated CLL patients who are unsuitable for full-dose Fludarabine-based therapy. This combination delays disease progression but incurs additional costs; thus, an assessment of its value for money is relevant. OBJECTIVE: To estimate the incremental cost-utility ratio of GClb in comparison with (i) rituximab in combination with chlorambucil (RClb), and (ii) chlorambucil alone (Clb) from the perspective of the Portuguese National Health Service (NHS). METHODS: A Markov model was used to predict disease progression. Pre-progression clinical data were based on the latest CLL11 trial data, and post-progression clinical data were obtained from CLL5 trial data. Utility values are from Kosmas et al. (Leuk Lymphoma 56:1320-1326, 14). Only direct medical costs were included. The resource consumption was estimated by a panel of Portuguese experts, and the unit costs were obtained from official sources. A discount rate of 5% was applied to costs and consequences. RESULTS: GClb and RClb were associated with an increase of 1.06 and 0.39 quality-adjusted life-years (QALY) at an additional cost of euro21,720 and euro9836 when compared to Clb, respectively. The cost-utility ratio of GClb versus Clb was euro20,397/QALY, while RClb was extendedly dominated. CONCLUSIONS: The use of GClb for previously untreated CLL patients who are unsuitable for full-dose Fludarabine-based therapy incurs an incremental cost per QALY that is generally accepted in Portugal. Therefore, although there is some uncertainty, obinutuzumab is probably a cost-effective therapy in the Portuguese setting.
Antitumor activity of fludarabine against human multiple myeloma in vitro and in vivo.[Pubmed:17976186]
Eur J Haematol. 2007 Dec;79(6):486-93.
Fludarabine, a nucleoside analogue, plays a major role in the treatment of B-cell lymphocytic leukemia, hairy cell leukemia, and indolent lymphomas. There is a controversy about antitumor activity of Fludarabine in multiple myeloma (MM). The aim of this study was to evaluate the activity of Fludarabine against human myeloma cells both in vivo and in vitro. We demonstrated that myeloma cell line RPMI8226 was efficiently inhibited by Fludarabine, concomitantly with decreased phosphorylation of Akt, down-regulation of the inhibitor of apoptosis proteins (IAP) family, including XIAP and survivin, and induction of apoptosis related to activation of caspase cascade. Contrary to dexamethasone, the effect of Fludarabine on RPMI8226 cells was independent of interleukin-6. Fludarabine also induced cytotoxicity in dexamethasone-sensitive (MM.1S) and -resistant (MM.1R) cells at 48 h with IC50 of 13.48 microg/mL and 33.79 microg/mL, respectively. In contrast, U266 cells were resistant to Fludarabine. Moreover, RPMI8226 myeloma xenograft model was established using severe combined immunodeficient mice. The tumors treated with Fludarabine at 40 mg/kg increased less than 5-fold in 25 d comparing with approximately 10-fold in the control tumors, demonstrating the antitumor activity of Fludarabine in vivo. These results suggest that Fludarabine may be an important therapeutic option for MM patients who are resistant to dexamethasone.
Fludarabine prevents smooth muscle proliferation in vitro and neointimal hyperplasia in vivo through specific inhibition of STAT-1 activation.[Pubmed:17293493]
Am J Physiol Heart Circ Physiol. 2007 Jun;292(6):H2935-43.
Drug-eluting stents are increasingly used to reduce in-stent restenosis and adverse cardiac events after percutaneous coronary interventions. However, the race for the ideal drug-eluting stent is still on, with special regard to the best stent-coating system and the most effective and less toxic drug. Fludarabine, a nucleoside analog, has both anti-inflammatory and antiproliferative cellular effects. The aim of the present study was to assess the cellular and molecular effects of Fludarabine on vascular smooth muscle cell (VSMC) growth in vitro and in vivo and the feasibility and efficacy of a Fludarabine-eluting stent. To study the biomolecular effects of Fludarabine on VSMC proliferation in vitro, rat VSMCs were grown in the presence of 50 microM Fludarabine or in the absence of the same. To evaluate the in vivo effect of this drug, male Wistar rats underwent balloon injury of the carotid artery, and Fludarabine was locally delivered at the time of injury. Finally, Fludarabine-eluting stents were in-laboratory manufactured and tested in a rabbit model of in-stent restenosis. Fludarabine markedly inhibited VSMC proliferation in cell culture. Furthermore, Fludarabine reduced neointimal formation after balloon angioplasty in a dose-dependent manner, and Fludarabine-eluting stents reduced neointimal hyperplasia by approximately 50%. These in vitro and in vivo cellular effects were specifically associated with the molecular switch-off of signal transducer and activator of transcription (STAT)-1 activation, without affecting other STAT proteins. Fludarabine abolishes VSMC proliferation in vitro and reduces neointimal formation after balloon injury in vivo through specific inhibition of STAT-1 activation. Fludarabine-eluting stents are feasible and effective in reducing in-stent restenosis in rabbits.
In vitro evaluation of fludarabine in combination with cyclophosphamide and/or mitoxantrone in B-cell chronic lymphocytic leukemia.[Pubmed:10515887]
Blood. 1999 Oct 15;94(8):2836-43.
B-chronic lymphocytic leukemia (B-CLL) is characterized by the accumulation of long-lived CD5(+) B lymphocytes. We have analyzed the effect in vitro of the combination of Fludarabine with cyclophosphamide and/or mitoxantrone on cells from 20 B-CLL patients. Mafosfamide, the active form of cyclophosphamide in vitro, increased the cytotoxicity of Fludarabine in all of the patients studied and produced a significant synergistic effect (P <.01) after 48 hours of incubation. The addition of mitoxantrone to this combination increased the cytotoxic effect in cells from 8 patients, but in the remaining 12 patients no significant increase was observed. The effect of Fludarabine and mafosfamide was dose-dependent. Mafosfamide and Fludarabine had a synergistic effect in inducing apoptosis of B-CLL cells as determined by DNA staining with propidium iodide and analysis of phosphatidylserine exposure. Mafosfamide significantly increased the apoptosis induced by Fludarabine on CD19(+) cells (P =.007), but not on CD3(+) cells (P =. 314). Cell viability was correlated with a decrease in Mcl-1 levels and an increase in p53 levels. These results support that Fludarabine in combination with cyclophosphamide and/or mitoxantrone can be highly effective in the treatment of B-CLL.
Fludarabine-induced immunosuppression is associated with inhibition of STAT1 signaling.[Pubmed:10202937]
Nat Med. 1999 Apr;5(4):444-7.
Fludarabine is a nucleoside analog used in the treatment of hematologic malignancies that can induce severe and prolonged immunosuppression. Although it can be incorporated into the DNA of dividing cells, Fludarabine is also a potent inhibitor of cells with a low growth fraction, thus it must have other mechanisms of action. STAT1, which is activated in response to many lymphocyte-activating cytokines including the interferons, is essential for cell-mediated immunity, as the absence of this protein is associated with prominent defects in the ability to control viral infections. Here we show that Fludarabine, but not the immunosuppressant cyclosporine A, inhibits the cytokine-induced activation of STAT1 and STAT1-dependent gene transcription in normal resting or activated lymphocytes. Fludarabine caused a specific depletion of STAT1 protein (and mRNA) but not of other STATs. This loss of STAT1 was also seen in cells from patients treated with Fludarabine in vivo. Brief exposure to Fludarabine led to a sustained loss of STAT1, analogous to the prolonged period of immunosuppression induced by exposure to the drug in vivo. Thus, STAT1 may be a useful target in the development of new immunosuppressive and antineoplastic agents.


