RWJ 21757Toll-like receptor 7 (TLR7) agonist; displays antitumor and antiviral activity CAS# 121288-39-9 |
- I-BET-762
Catalog No.:BCC4474
CAS No.:1260907-17-2
- Bromodomain Inhibitor, (+)-JQ1
Catalog No.:BCC1132
CAS No.:1268524-70-4
- I-BET151 (GSK1210151A)
Catalog No.:BCC4476
CAS No.:1300031-49-5
- GSK1324726A
Catalog No.:BCC4038
CAS No.:1300031-52-0
- PFI-1 (PF-6405761)
Catalog No.:BCC2225
CAS No.:1403764-72-6
Quality Control & MSDS
Number of papers citing our products

Chemical structure

3D structure
| Cas No. | 121288-39-9 | SDF | Download SDF |
| PubChem ID | 60737 | Appearance | Powder |
| Formula | C13H17N5O6 | M.Wt | 339.3 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
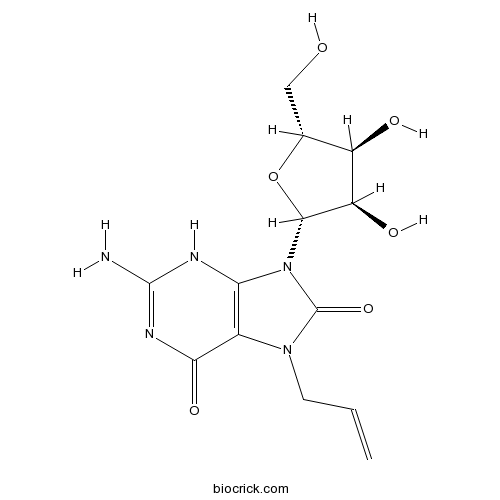
| Synonyms | Loxoribine, 7-Allyl-8-oxoguanosine | ||
| Solubility | Soluble to 20 mM in water and to 75 mM in DMSO | ||
| Chemical Name | 2-amino-9-[(2R,3R,4S,5R)-3,4-dihydroxy-5-(hydroxymethyl)oxolan-2-yl]-7-prop-2-enyl-3H-purine-6,8-dione | ||
| SMILES | C=CCN1C2=C(NC(=NC2=O)N)N(C1=O)C3C(C(C(O3)CO)O)O | ||
| Standard InChIKey | VDCRFBBZFHHYGT-IOSLPCCCSA-N | ||
| Standard InChI | InChI=1S/C13H17N5O6/c1-2-3-17-6-9(15-12(14)16-10(6)22)18(13(17)23)11-8(21)7(20)5(4-19)24-11/h2,5,7-8,11,19-21H,1,3-4H2,(H3,14,15,16,22)/t5-,7-,8-,11-/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Toll-like receptor 7 (TLR7) agonist; induces immune cell activation and increases cytokine production. Displays antitumor and antiviral activity in various animal models. |

RWJ 21757 Dilution Calculator

RWJ 21757 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.9472 mL | 14.7362 mL | 29.4724 mL | 58.9449 mL | 73.6811 mL |
| 5 mM | 0.5894 mL | 2.9472 mL | 5.8945 mL | 11.789 mL | 14.7362 mL |
| 10 mM | 0.2947 mL | 1.4736 mL | 2.9472 mL | 5.8945 mL | 7.3681 mL |
| 50 mM | 0.0589 mL | 0.2947 mL | 0.5894 mL | 1.1789 mL | 1.4736 mL |
| 100 mM | 0.0295 mL | 0.1474 mL | 0.2947 mL | 0.5894 mL | 0.7368 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Alendronate
Catalog No.:BCC4885
CAS No.:121268-17-5
- ICI 204,448 hydrochloride
Catalog No.:BCC6806
CAS No.:121264-04-8
- Calphostin C
Catalog No.:BCC7131
CAS No.:121263-19-2
- Secodihydro-hydramicromelin B
Catalog No.:BCN4783
CAS No.:1212148-58-7
- [Ala1,3,11,15]-Endothelin
Catalog No.:BCC5731
CAS No.:121204-87-3
- TC-N 1752
Catalog No.:BCC6179
CAS No.:1211866-85-1
- Rauvoyunine C
Catalog No.:BCN4833
CAS No.:1211543-01-9
- LEE011 hydrochloride
Catalog No.:BCC4101
CAS No.:1211443-80-9
- LEE011
Catalog No.:BCC3926
CAS No.:1211441-98-3
- Cefprozil hydrate
Catalog No.:BCC4951
CAS No.:121123-17-9
- EG00229
Catalog No.:BCC5376
CAS No.:1210945-69-9
- L-670,596
Catalog No.:BCC5857
CAS No.:121083-05-4
- 1-Hydroxybisabola-2,10-dien-4-one
Catalog No.:BCN7297
CAS No.:1213251-45-6
- WZ4002
Catalog No.:BCC1074
CAS No.:1213269-23-8
- Fmoc-Glu-OH
Catalog No.:BCC3489
CAS No.:121343-82-6
- SR 33805 oxalate
Catalog No.:BCC7181
CAS No.:121346-33-6
- Bernardioside A
Catalog No.:BCN7862
CAS No.:121368-52-3
- N6-Benzyladenine
Catalog No.:BCC9076
CAS No.:1214-39-7
- POM 1
Catalog No.:BCC7454
CAS No.:12141-67-2
- WZ3146
Catalog No.:BCC4004
CAS No.:1214265-56-1
- WZ8040
Catalog No.:BCC1075
CAS No.:1214265-57-2
- WZ4003
Catalog No.:BCC4363
CAS No.:1214265-58-3
- Ajugapantin A
Catalog No.:BCN3663
CAS No.:121449-67-0
- Daclatasvir (BMS-790052)
Catalog No.:BCC2533
CAS No.:1214735-16-6
Identification of an active metabolite of PAR-1 antagonist RWJ-58259 and synthesis of analogues to enhance its metabolic stability.[Pubmed:26927018]
Org Biomol Chem. 2016 Mar 28;14(12):3198-201.
The discontinuation of PAR-1 antagonist RWJ-58259 beyond use as a biological probe is most likely due to it's short half-life in vivo. However, retention of significant in vivo activity beyond the point where most of the RWJ-58259 had been consumed implies the generation of an active metabolite. Herein we describe the biological activity of a predicted metabolite of RWJ-58259 and the synthesis of analogues designed to enhance the metabolic stability of RWJ-58259.
Leveraging Academic-Service Partnerships: Implications for Implementing the RWJ/IOM's Recommendations to Improve Quality, Access, and Value in Academic Medical Centers.[Pubmed:22191053]
ISRN Nurs. 2011;2011:731902.
Transformation of the current healthcare system is critical to achieve improved quality, safety, value, and access. Patients with multiple, chronic health conditions require integrated care coordination yet the current health care system is fragmented and complex. Nursing must play a key role in constructing a system that is value based and patient focused. The Robert Wood Johnson/Institute of Medicine (RWJ/IOM) report on the future of nursing outlines strategic opportunities for nursing to take a lead role in this transformation. Partnerships across academic institutions and health care systems have the potential to address issues through mutual goal setting, sharing of risks, responsibilities, and accountability, and realignment of resources. The purpose of this paper is to present Stony Brook University Medical Center's (SBUMC) academic-service partnership which implemented several of the RWJ/IOM recommendations. The partnership resulted in several initiatives that improved quality, safety, access, and value. It also characterized mutual goal setting, shared missions and values, and a united vision for health care.
Synergistic Effects of Transplanted Endothelial Progenitor Cells and RWJ 67657 in Diabetic Ischemic Stroke Models.[Pubmed:26045601]
Stroke. 2015 Jul;46(7):1938-46.
BACKGROUND AND PURPOSE: An immature vascular phenotype in diabetes mellitus may cause more severe vascular damage and poorer functional outcomes after stroke, and it would be feasible to repair damaged functional vessels using endothelial progenitor cell (EPC) transplantation. However, high glucose induces p38 mitogen-activated protein kinase activation, which can accelerate the senescence and apoptosis of EPCs. The aim of this study was to investigate the combined effects of EPC transplantation and p38 mitogen-activated protein kinase inhibitor administration on diabetic stroke outcomes. METHODS: Bone marrow-derived EPCs were injected intra-arterially into db/db mice after ischemic stroke induction. RWJ 67657 (RWJ), a p38 mitogen-activated protein kinase inhibitor, was administered orally for 7 consecutive days, with the first dose given 30 minutes before stroke induction. Functional outcome was determined at days 0, 1, 7, 14, and 21. Angiogenesis, neurogenesis, infarct volume, and Western blotting assays were performed on day 7, and white matter remodeling was determined on day 14. RESULTS: Neither EPC transplantation nor RWJ administration alone significantly improved diabetic stroke outcome although RWJ displayed a potent anti-inflammatory effect. By both improving the functioning of EPCs and reducing inflammation, EPC transplantation plus RWJ administration in vivo synergistically promoted angiogenesis and neurogenesis after diabetic stroke. In addition, the white matter remodeling, behavioral scores, and expressions of vascular endothelial growth factor and brain-derived neurotrophic factor were significantly increased in diabetic mice treated with both EPCs and RWJ. CONCLUSIONS: The combination of EPC transplantation and RWJ administration accelerated recovery from diabetic stroke, which might have been caused by increased levels of proangiogenic and neurotrophic factors.
The Toll-like receptor 7 (TLR7)-specific stimulus loxoribine uncovers a strong relationship within the TLR7, 8 and 9 subfamily.[Pubmed:14579267]
Eur J Immunol. 2003 Nov;33(11):2987-97.
Loxoribine (7-allyl-7,8-dihydro-8-oxo-guanosine) acts as synthetic adjuvant in anti-tumor responses. Here we first demonstrate that loxoribine activates cells of the innate immune system selectively via the Toll-like receptor (TLR) 7/MyD88-dependent signaling pathway. TLR7- and MyD88-deficient immune cells fail to proliferate or produce cytokines in response to loxoribine, and genetic complementation of TLR7-deficient cells with murine or human TLR7 confers responsiveness. Subsequently we show that cellular activation by loxoribine and resiquimod (R-848), a stimulus for TLR7 and TLR8, depends on acidification and maturation of endosomes and targets MyD88 to vesicular structures with lysosomal characteristics. This mode of TLR7 and TLR8 action resembles CpG-DNA-driven TLR9 activation. We thus conclude that TLR7, 8 and 9 form a functional subgroup within the TLR family that recognizes pathogen-associated molecular patterns in endosomal/lysosomal compartments.
Molecular basis for the immunostimulatory activity of guanine nucleoside analogs: activation of Toll-like receptor 7.[Pubmed:12738885]
Proc Natl Acad Sci U S A. 2003 May 27;100(11):6646-51.
Certain C8-substituted and N7, C8-disubstituted guanine ribonucleosides comprise a class of small molecules with immunostimulatory activity. In a variety of animal models, these agents stimulate both humoral and cellular immune responses. The antiviral actions of these guanosine analogs have been attributed to their ability to induce type I IFNs. However, the molecular mechanisms by which the guanosine analogs potentiate immune responses are not known. Here, we report that several guanosine analogs activate Toll-like receptor 7 (TLR7). 7-Thia-8-oxoguanosine, 7-deazaguanosine, and related guanosine analogs activated mouse immune cells in a manner analogous to known TLR ligands, inducing cytokine production in mouse splenocytes (IL-6 and IL-12, type I and II IFNs), bone marrow-derived macrophages (IL-6 and IL-12), and in human peripheral blood leukocytes (type I IFNs, tumor necrosis factor alpha and IL-12). The guanosine congeners also up-regulated costimulatory molecules and MHC I/II in dendritic cells. Genetic complementation studies in human embryonic kidney 293 cells confirmed that the guanosine analogs activate cells exclusively via TLR7. The stimulation of TLR7 by the guanosine analogs in human cells appears to require endosomal maturation because inhibition of this process with chloroquine significantly reduced the downstream activation of NF-kappaB. However, TLR8 activation by R-848 and TLR2 activation by [S-[2,3-bis(palmitoyloxy)-(2-RS)-propyl]-N-palmitoyl-R-Cys-S-Ser-Lys4-OH, trihydrochloride)] were not inhibited by chloroquine, whereas TLR9 activation by CpG oligodeoxynucleotides was abolished. In summary, we present evidence that guanosine analogs activate immune cells via TLR7 by a pathway that requires endosomal maturation. Thus, the B cell-stimulating and antiviral activities of the guanosine analogs may be explained by their TLR7-activating capacity.
Loxoribine induces chronic lymphocytic leukemia B cells to traverse the cell cycle.[Pubmed:7949100]
Blood. 1994 Nov 15;84(10):3457-64.
Leukemic B cells from a majority of patients with chronic lymphocytic leukemia (CLL) enter the cell cycle upon stimulation in vitro with loxoribine, a potent 7,8-disubstituted guanine ribonucleoside immunostimulant. In the absence of added costimulants, a proportion of these cells become activated and undergo DNA synthesis and mitosis accompanied by a marked increase in expression of an array of cell surface activation antigens. The resultant activated B-CLL cells exhibit greatly enhanced sensitivity to cycle-active cytotoxic drugs. This approach may be of potential value in the therapy of CLL.


