SBHAHDAC1/HDAC3 inhibitor,cell-permeable CAS# 38937-66-5 |
- CUDC-101
Catalog No.:BCC2149
CAS No.:1012054-59-9
- CI994 (Tacedinaline)
Catalog No.:BCC2159
CAS No.:112522-64-2
- Romidepsin (FK228, depsipeptide)
Catalog No.:BCC3597
CAS No.:128517-07-7
- Pyroxamide
Catalog No.:BCC2424
CAS No.:382180-17-8
- Valproic acid
Catalog No.:BCC4260
CAS No.:99-66-1
- Droxinostat
Catalog No.:BCC2157
CAS No.:99873-43-5
Quality Control & MSDS
Number of papers citing our products

Chemical structure

3D structure
| Cas No. | 38937-66-5 | SDF | Download SDF |
| PubChem ID | 5173 | Appearance | Powder |
| Formula | C8H16N2O4 | M.Wt | 204.22 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 100 mM in water and to 100 mM in DMSO | ||
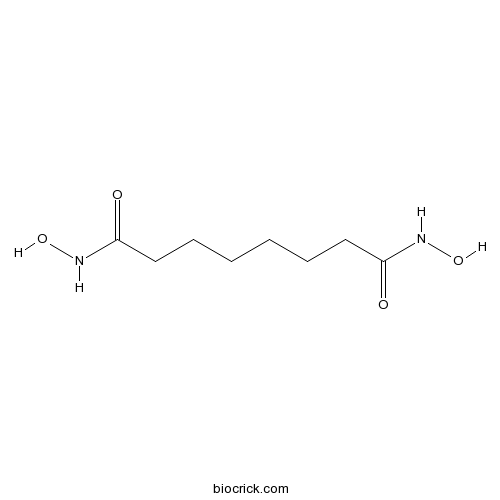
| Chemical Name | N,N'-dihydroxyoctanediamide | ||
| SMILES | C(CCCC(=O)NO)CCC(=O)NO | ||
| Standard InChIKey | IDQPVOFTURLJPT-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C8H16N2O4/c11-7(9-13)5-3-1-2-4-6-8(12)10-14/h13-14H,1-6H2,(H,9,11)(H,10,12) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Histone deacetylase (HDAC) inhibitor (ID50 values are 0.25 and 0.3 μM for HDAC1 and HDAC3 respectively). Potentiates the cytostatic effects of 5-Fluorouracil in colorectal cancer cells. |

SBHA Dilution Calculator

SBHA Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 4.8967 mL | 24.4834 mL | 48.9668 mL | 97.9336 mL | 122.417 mL |
| 5 mM | 0.9793 mL | 4.8967 mL | 9.7934 mL | 19.5867 mL | 24.4834 mL |
| 10 mM | 0.4897 mL | 2.4483 mL | 4.8967 mL | 9.7934 mL | 12.2417 mL |
| 50 mM | 0.0979 mL | 0.4897 mL | 0.9793 mL | 1.9587 mL | 2.4483 mL |
| 100 mM | 0.049 mL | 0.2448 mL | 0.4897 mL | 0.9793 mL | 1.2242 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
SBHA (suberohydroxamic acid) is a Histone deacetylase (HDAC) inhibitor, ID50 for HDAC1 and HDAC3 are 0.25 and 0.3 μM, respectively.
HDAC is an enzyme that deacetylates lysine residues on the N-terminal of the core histones. It is required for transcriptional modulation, cell cycle regulation and development.
In MEL cells, SBHA leads to the accumulation of acetylated H4, indicating the hydroxamic acid groups are involved in increasing potency and inhibition of HDAC activity. In Jurkat cells, SHBA inhibits the HDAC1 and HDAC3 activities with IC50 value of 0.25 and 0.3 μm respectively. [1]
In melanoma cell lines, SBHA also induces apoptosis and mitochondrial membrane permeability in a caspase-dependent manner. SBHA down-regulates main antiapoptotic proteins such as Bcl-XL and Mcl-1 and up-regulates proapoptic proteins such as Bim, Bax and Bak. In addition, SBHA induces translocation of Bim to mitochordria and its association with Bcl-2. [2]
References:
1. Richon VM, Emiliani S, Verdin E et al. A class of hybrid polar inducers of transformed cell differentiation inhibits histone deacetylases. Proc Natl Acad Sci U S A. 1998 Mar 17; 95(6):3003-7.
2. Zhang XD, Gillespie SK, Borrow JM, Hersey P. The histone deacetylase inhibitor suberic bishydroxamate regulates the expression of multiple apoptotic mediators and induces mitochondria-dependent apoptosis of melanoma cells. Mol Cancer Ther. 2004 Apr; 3(4):425-35.
- Isodeoxyelephantopin
Catalog No.:BCN4638
CAS No.:38927-54-7
- erythro-Guaiacylglycerol
Catalog No.:BCN5440
CAS No.:38916-91-5
- Halaminol C
Catalog No.:BCN1787
CAS No.:389125-68-2
- Halaminol B
Catalog No.:BCN1748
CAS No.:389125-59-1
- Halaminol A
Catalog No.:BCN1788
CAS No.:389125-56-8
- Z-Glu(OtBu)-OH
Catalog No.:BCC2776
CAS No.:3886-08-6
- CK 869
Catalog No.:BCC6347
CAS No.:388592-44-7
- PSN 375963 hydrochloride
Catalog No.:BCC7663
CAS No.:388575-52-8
- Tetrahydroepiberberine
Catalog No.:BCN2649
CAS No.:38853-67-7
- Emodin-1-O-glucoside
Catalog No.:BCN8512
CAS No.:38840-23-2
- Lapatinib Ditosylate
Catalog No.:BCC2083
CAS No.:388082-78-8
- Tandutinib (MLN518)
Catalog No.:BCC4499
CAS No.:387867-13-2
- Chelerythrine chloride
Catalog No.:BCN8322
CAS No.:3895-92-9
- Vitexin 4'-glucoside
Catalog No.:BCC9252
CAS No.:38950-94-6
- Isovitexin
Catalog No.:BCN5441
CAS No.:38953-85-4
- Icariside D2
Catalog No.:BCN7217
CAS No.:38954-02-8
- Piperlotine A
Catalog No.:BCN6481
CAS No.:389572-70-7
- Prasugrel hydrochloride
Catalog No.:BCC4291
CAS No.:389574-19-0
- Prasugrel Maleic acid
Catalog No.:BCC4292
CAS No.:389574-20-3
- Raspberry ketone glucoside
Catalog No.:BCC8244
CAS No.:38963-94-9
- N-Acetylnorloline
Catalog No.:BCN2005
CAS No.:38964-35-1
- Eriodictyol-7-O-glucoside
Catalog No.:BCN4743
CAS No.:38965-51-4
- 2'-O-Methylperlatolic acid
Catalog No.:BCN5442
CAS No.:38968-07-9
- Ophiopogonin B
Catalog No.:BCN5378
CAS No.:38971-41-4
HDAC inhibitors, MS275 and SBHA, enhances cytotoxicity induced by oxaliplatin in the colorectal cancer cell lines.[Pubmed:19596269]
Biochem Biophys Res Commun. 2009 Sep 18;387(2):336-41.
Histone deacetylases (HDACs) play an important role in the epigenetic regulation of gene expression by catalyzing the removal of acetyl groups, stimulating chromatin condensation, and promoting transcriptional repression. Since aberrant epigenetic changes are a hallmark of cancer, HDACs appear a promising target for pharmacological inhibition. To test this we have addressed the hypothesis that histone deacetylase inhibitors (HDACi), MS275 and SBHA, potentiate inhibitory effects of classical anti-colorectal cancer cytostatic, oxaliplatin, on survival of colorectal cancer (CRC) cells in vitro. We are reporting here that HDACi shows potent synergistic interaction with oxaliplatin. The observed synergism between HDACi and oxaliplatin is most probably related to the augmented apoptotic signal and allowed for significant reduction of doses of anticancer agents used.
Bim plays a crucial role in synergistic induction of apoptosis by the histone deacetylase inhibitor SBHA and TRAIL in melanoma cells.[Pubmed:17051334]
Apoptosis. 2006 Dec;11(12):2251-65.
The wide variation in sensitivity of cancer cells to TRAIL- or histone deacetylase (HDAC) inhibitor - induced apoptosis precludes successful treatment of cancer with these agents. We report here that TRAIL and SBHA synergistically induce apoptosis of melanoma cells as revealed by quantitative analysis using the normalized isobologram method. This is supported by enhanced activation of caspase-3 and cleavage of its substrates, PARP and ICAD. Co-treatment with SBHA and TRAIL did not enhance formation of the death-inducing signaling complex (DISC) and processing of caspase-8 and Bid, but potentiated activation of Bax and release of Cytochrome C and Smac/DIABLO from mitochondria into the cytosol. SBHA down-regulated Bcl-X(L), Mcl-1 and XIAP, but up-regulated Bax, Bak, and the BH3-only protein Bim(EL). Up-regulation of the latter by SBHA was attenuated by the presence of TRAIL, which was inhibitable by the pan-caspase inhibitor z-VAD-fmk. Inhibition of Bim by siRNA attenuated conformational changes of Bax, mitochondrial apoptotic events, and activation of caspase-3, leading to marked inhibition of the synergy between SBHA and TRAIL. Thus, Bim plays an essential role in synergistic induction of apoptosis by SBHA and TRAIL in melanoma.
The histone deacetylase inhibitor suberic bishydroxamate regulates the expression of multiple apoptotic mediators and induces mitochondria-dependent apoptosis of melanoma cells.[Pubmed:15078986]
Mol Cancer Ther. 2004 Apr;3(4):425-35.
Histone deacetylase (HDAC) inhibitors have attracted much interest because of their ability to arrest cell growth, induce cell differentiation, and in some cases, induce apoptosis of cancer cells. In the present study, we have examined a new HDAC inhibitor, suberic bishydroxamate (SBHA), for its effect on a panel of human melanoma cell lines. We report that it induces varying degrees of apoptosis in the melanoma lines but not in melanocytes and fibroblasts. Induction of apoptosis was caspase dependent and was associated with induction of changes in mitochondrial membrane permeability, which could be inhibited by overexpression of Bcl-2. The changes in mitochondria were independent of caspase activation and were associated with changes in conformation of Bax. SBHA down-regulated several key antiapoptotic proteins including X-linked inhibitor of apoptosis and the Bcl-2 family proteins, Bcl-XL and Mcl-1. In contrast, it induced up-regulation of the Bcl-2 family proapoptotic proteins, Bim, Bax, and Bak. In addition, SBHA induced relocation of the protein Bim to mitochondria and its association with Bcl-2. De novo protein synthesis was required for initiation of apoptosis in that the protein synthesis inhibitor, cycloheximide, inhibited SBHA-induced conformational changes in Bax as well as changes in mitochondrial membrane permeability and activation of caspase-3. These results suggest that SBHA induces apoptosis by changing the balance between proapoptotic and antiapoptotic proteins in melanoma cells. The protein Bim may be a key initiator of apoptosis in cells treated with SBHA.
Histone hyperacetylation induced by histone deacetylase inhibitors is not sufficient to cause growth inhibition in human dermal fibroblasts.[Pubmed:11304533]
J Biol Chem. 2001 Jun 22;276(25):22491-9.
Use of specific histone deacetylase inhibitors has revealed critical roles for the histone deacetylases (HDAC) in controlling proliferation. Although many studies have correlated the function of HDAC inhibitors with the hyperacetylation of histones, few studies have specifically addressed whether the accumulation of acetylated histones, caused by HDAC inhibitor treatment, is responsible for growth inhibition. In the present study we show that HDAC inhibitors cause growth inhibition in normal and transformed keratinocytes but not in normal dermal fibroblasts. This was despite the observation that the HDAC inhibitor, suberic bishydroxamate (SBHA), caused a kinetically similar accumulation of hyperacetylated histones. This cell type-specific response to SBHA was not due to the inactivation of SBHA by fibroblasts, nor was it due to differences in the expression of specific HDAC family members. Remarkably, overexpression of HDACs 1, 4, and 6 in normal human fibroblasts resulted in cells that could be growth-inhibited by SBHA. These data suggest that, although histone acetylation is a major target for HDAC inhibitors, the accumulation of hyperacetylated histones is not sufficient to cause growth inhibition in all cell types. This suggests that growth inhibition, caused by HDAC inhibitors, may be the culmination of histone hyperacetylation acting in concert with other growth regulatory pathways.
A class of hybrid polar inducers of transformed cell differentiation inhibits histone deacetylases.[Pubmed:9501205]
Proc Natl Acad Sci U S A. 1998 Mar 17;95(6):3003-7.
Hybrid polar compounds (HPCs) have been synthesized that induce terminal differentiation and/or apoptosis in various transformed cells. We have previously reported on the development of the second-generation HPCs suberoylanilide hydroxamic acid (SAHA) and m-carboxycinnamic acid bishydroxamide (CBHA) that are 2,000-fold more potent inducers on a molar basis than the prototype HPC hexamethylene bisacetamide (HMBA). Herein we report that CBHA and SAHA inhibit histone deacetylase 1 (HDAC1) and histone deacetylase 3 (HDAC3) activity in vitro. Treatment of cells in culture with SAHA results in a marked hyperacetylation of histone H4, but culture with HMBA does not. Murine erythroleukemia cells developed for resistance to SAHA are cross-resistant to trichostatin A, a known deacetylase inhibitor and differentiation inducer, but are not cross-resistant to HMBA. These studies show that the second-generation HPCs, unlike HMBA, are potent inhibitors of HDAC activity. In this sense, HMBA and the second-generation HPCs appear to induce differentiation by different pathways.


