Sal 003Cellular phosphatase complex inhibitor CAS# 1164470-53-4 |
- Calyculin A
Catalog No.:BCC2457
CAS No.:101932-71-2
- Calcineurin Autoinhibitory Peptide
Catalog No.:BCC2456
CAS No.:148067-21-4
- DL-AP3
Catalog No.:BCC2459
CAS No.:20263-06-3
- Ceramide
Catalog No.:BCC2458
CAS No.:3102-57-6
- Fostriecin sodium salt
Catalog No.:BCC2460
CAS No.:87860-39-7
Quality Control & MSDS
Number of papers citing our products

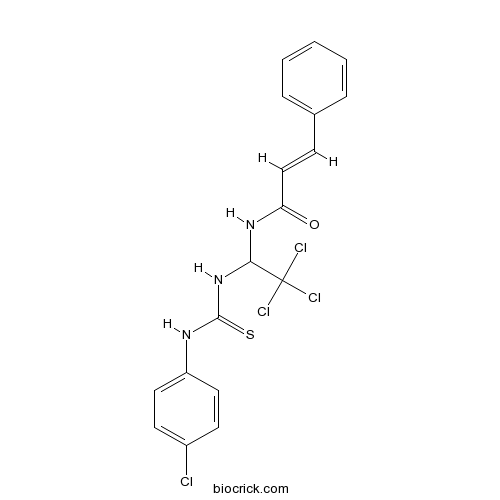
Chemical structure
3D structure
| Cas No. | 1164470-53-4 | SDF | Download SDF |
| PubChem ID | 5717737 | Appearance | Powder |
| Formula | C18H15Cl4N3OS | M.Wt | 463.21 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 100 mM in DMSO | ||
| Chemical Name | (E)-3-phenyl-N-[2,2,2-trichloro-1-[(4-chlorophenyl)carbamothioylamino]ethyl]prop-2-enamide | ||
| SMILES | C1=CC=C(C=C1)C=CC(=O)NC(C(Cl)(Cl)Cl)NC(=S)NC2=CC=C(C=C2)Cl | ||
| Standard InChIKey | TVNBASWNLOIQML-IZZDOVSWSA-N | ||
| Standard InChI | InChI=1S/C18H15Cl4N3OS/c19-13-7-9-14(10-8-13)23-17(27)25-16(18(20,21)22)24-15(26)11-6-12-4-2-1-3-5-12/h1-11,16H,(H,24,26)(H2,23,25,27)/b11-6+ | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Cell-permeable inhibitor of cellular phosphatase complexes that dephosphorylate eukaryotic translation initiation factor 2 subunit α (eIF2α). Analog of salubrinal with improved aqueous solubility. Shown to prevent the induction of hippocampal long-term potentiation (LTP) and memory formation (LTM) in mice. |

Sal 003 Dilution Calculator

Sal 003 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.1588 mL | 10.7942 mL | 21.5885 mL | 43.177 mL | 53.9712 mL |
| 5 mM | 0.4318 mL | 2.1588 mL | 4.3177 mL | 8.6354 mL | 10.7942 mL |
| 10 mM | 0.2159 mL | 1.0794 mL | 2.1588 mL | 4.3177 mL | 5.3971 mL |
| 50 mM | 0.0432 mL | 0.2159 mL | 0.4318 mL | 0.8635 mL | 1.0794 mL |
| 100 mM | 0.0216 mL | 0.1079 mL | 0.2159 mL | 0.4318 mL | 0.5397 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Eukaryotic initiation factor 2 α-subunit (eIF2α) plays a critical role in the regulation of protein synthesis. Moreover, it also plays an important role in synaptic plasticity and long-term memory consolidation. Sal003 is a selective inhibitor of eIF2α dephosphorylation.
In vitro: Sal003 specifically prevents dephosphorylation of eIF2α by blocking eIF2α phosphatases. In Sal003-treated cells polysomes dissociated and consistent with this β-actin mRNA shifted to lighter fractions, reflecting the inhibition of general translation [1].
In vivo: Intra-basolateral amygdala (BLA) infusions of Sal003 immediately after memory retrieval disrupted the reconsolidation of morphine- or cocaine-induced CPP, leading to a long-lasting suppression of drug-paired stimulus-induced craving. Moreover, inhibition of eIF2α dephosphorylation in the BLA immediately after light/tone stimulus retrieval decreased subsequent cue-induced heroin-seeking behavior in the self-administration procedure. These results demonstrate that eIF2α dephosphorylation in the BLA mediates the memory reconsolidation of drug-paired stimuli [1].
Clinical trials: Currenlty no clinical data are available.
Reference:
[1] Jian M, Luo YX, Xue YX, Han Y, Shi HS, Liu JF, Yan W, Wu P, Meng SQ, Deng JH, Shen HW, Shi J, Lu L. eIF2α dephosphorylation in basolateral amygdala mediates reconsolidation of drug memory. J Neurosci. 2014;34(30):10010-21.
- Curcumadione
Catalog No.:BCN3525
CAS No.:116425-36-6
- Aerugidiol
Catalog No.:BCN3529
CAS No.:116425-35-5
- Fargesone B
Catalog No.:BCN6415
CAS No.:116424-70-5
- Fargesone A
Catalog No.:BCN6417
CAS No.:116424-69-2
- 9S-10alpha-Hydroxyepigambogic acid
Catalog No.:BCN3080
CAS No.:1164201-85-7
- SR 3576
Catalog No.:BCC7999
CAS No.:1164153-22-3
- Androstanolone acetate
Catalog No.:BCC8826
CAS No.:1164-91-6
- Z-Tyr-OH
Catalog No.:BCC2747
CAS No.:1164-16-5
- 3',4',7-Trimethoxyflavan
Catalog No.:BCN6042
CAS No.:116384-26-0
- Loureirin C
Catalog No.:BCN3761
CAS No.:116384-24-8
- 2alpha-hydroxy-3beta-acetyloxy-betulic acid
Catalog No.:BCN3072
CAS No.:1163728-89-9
- Acetylexidonin
Catalog No.:BCN3279
CAS No.:116368-90-2
- ML 145
Catalog No.:BCC7876
CAS No.:1164500-72-4
- 5,5'-Dimethoxylariciresinol
Catalog No.:BCN6043
CAS No.:116498-58-9
- 9alpha,13alpha-Epidioxyabiet-8(14)-en-18-oic acid
Catalog No.:BCN1611
CAS No.:116499-73-1
- Aflatoxin G1
Catalog No.:BCC9214
CAS No.:1165-39-5
- Dehydroalisol B 23-acetate
Catalog No.:BCC9240
CAS No.:
- 3-Methylamino-1-(2-thienyl)-1-propanol
Catalog No.:BCC8636
CAS No.:116539-55-0
- A66
Catalog No.:BCC3715
CAS No.:1166227-08-2
- Mibefradil
Catalog No.:BCC1748
CAS No.:116644-53-2
- Mibefradil dihydrochloride
Catalog No.:BCC1749
CAS No.:116666-63-8
- Mycophenolate mofetil hydrochloride
Catalog No.:BCC4117
CAS No.:116680-01-4
- AZD7687
Catalog No.:BCC1394
CAS No.:1166827-44-6
- FK 3311
Catalog No.:BCC1576
CAS No.:116686-15-8
Real-Life and RCT Participants: Alendronate Users Versus FITs' Trial Eligibility Criterion.[Pubmed:27099132]
Calcif Tissue Int. 2016 Sep;99(3):243-9.
We aimed to characterize incident users of alendronate from Denmark and Spain, and investigate their eligibility for participation in the pivotal Fracture Intervention Trial (FIT). This is an international cross-sectional study, where the data were obtained from the SIDIAP database (Sistema d'Informacio per al Desenvolupament de l'Investigacio en Atencio Primaria) from Catalonia (Spain) and the Danish Health Registries (DHR). This study included patients who were incident users of alendronate, >/=40 years old with no history of Paget's disease. Our measurements were the proportion of incident users of alendronate who were not eligible to participate in FIT. 14,316 and 21,221 subjects initiated alendronate in 2006-2007 (SIDIAP) and 2005-2006 (DHR), respectively. SIDIAP and DHR alendronate user cohorts had 2347 (16.4 %) and 5275 (24.9 %) subjects aged >80 years old, reported 9 (0.1 %) and 91 (0.4 %) diagnoses of myocardial infarction, 423 (3 %) and 368 (1.7 %) of erosive gastro-intestinal disease, 200 (1.4 %) and 1109 (5.2 %) of dyspepsia, and 349 (2.4 %) and 149 (0.7 %) of metabolic bone disease, all of which were exclusion criteria in FIT. Men [3818 (26.7 %) in SIDIAP and 3885 (18.3 %) in DHR] and glucocorticoid users [1229 (8.6 %) in SIDIAP and 4716 (22.2 %) in DHR] were also excluded from the FIT trial. Overall, 3447 (35.4 %) SIDIAP and 6228 (44.5 %) (when not considering men and glucocorticoid users) DHR of incident alendronate users would have been excluded from FIT. One in two real-life users of alendronate exhibited one or more clinical characteristics that would have led to them being excluded from the FIT trial.
Variable ventilation improves pulmonary function and reduces lung damage without increasing bacterial translocation in a rat model of experimental pneumonia.[Pubmed:27887604]
Respir Res. 2016 Nov 25;17(1):158.
BACKGROUND: Variable ventilation has been shown to improve pulmonary function and reduce lung damage in different models of acute respiratory distress syndrome. Nevertheless, variable ventilation has not been tested during pneumonia. Theoretically, periodic increases in tidal volume (VT) and airway pressures might worsen the impairment of alveolar barrier function usually seen in pneumonia and could increase bacterial translocation into the bloodstream. We investigated the impact of variable ventilation on lung function and histologic damage, as well as markers of lung inflammation, epithelial and endothelial cell damage, and alveolar stress, and bacterial translocation in experimental pneumonia. METHODS: Thirty-two Wistar rats were randomly assigned to receive intratracheal of Pseudomonas aeruginosa (PA) or saline (SAL) (n = 16/group). After 24-h, animals were anesthetized and ventilated for 2 h with either conventional volume-controlled (VCV) or variable volume-controlled ventilation (VV), with mean VT = 6 mL/kg, PEEP = 5cmH2O, and FiO2 = 0.4. During VV, tidal volume varied randomly with a coefficient of variation of 30% and a Gaussian distribution. Additional animals assigned to receive either PA or SAL (n = 8/group) were not ventilated (NV) to serve as controls. RESULTS: In both SAL and PA, VV improved oxygenation and lung elastance compared to VCV. In SAL, VV decreased interleukin (IL)-6 expression compared to VCV (median [interquartile range]: 1.3 [0.3-2.3] vs. 5.3 [3.6-7.0]; p = 0.02) and increased surfactant protein-D expression compared to NV (2.5 [1.9-3.5] vs. 1.2 [0.8-1.2]; p = 0.0005). In PA, compared to VCV, VV reduced perivascular edema (2.5 [2.0-3.75] vs. 6.0 [4.5-6.0]; p < 0.0001), septum neutrophils (2.0 [1.0-4.0] vs. 5.0 [3.3-6.0]; p = 0.0008), necrotizing vasculitis (3.0 [2.0-5.5] vs. 6.0 [6.0-6.0]; p = 0.0003), and ultrastructural lung damage scores (16 [14-17] vs. 24 [14-27], p < 0.0001). Blood colony-forming-unit (CFU) counts were comparable (7 [0-28] vs. 6 [0-26], p = 0.77). Compared to NV, VCV, but not VV, increased expression amphiregulin, IL-6, and cytokine-induced neutrophil chemoattractant (CINC)-1 (2.1 [1.6-2.5] vs. 0.9 [0.7-1.2], p = 0.025; 12.3 [7.9-22.0] vs. 0.8 [0.6-1.9], p = 0.006; and 4.4 [2.9-5.6] vs. 0.9 [0.8-1.4], p = 0.003, respectively). Angiopoietin-2 expression was lower in VV compared to NV animals (0.5 [0.3-0.8] vs. 1.3 [1.0-1.5], p = 0.01). CONCLUSION: In this rat model of pneumonia, VV improved pulmonary function and reduced lung damage as compared to VCV, without increasing bacterial translocation.
The relationship of salivary testosterone and male sexual dysfunction in opioid-associated androgen deficiency (OPIAD).[Pubmed:27750480]
Aging Male. 2017 Mar;20(1):1-8.
BACKGROUND: Opioids are an effective treatment for chronic non-malignant pain (CNP). Long-term use risks and side effects such as opioid-induced androgen deficiency (OPIAD) exist. This could be measured by saliva testosterone (Sal-T). OBJECTIVES: To evaluate OPIAD in long-term opioid use in CNP patients. METHODS: A cross-sectional study included CNP male outpatients under opioid treatment. Total-Testosterone (Total-T), Free-Testosterone (Free-T), Bio-Testosterone (Bio-T) and Sal-T were measured. Correlations were calculated by Spearman's rho (SPSS 20). RESULTS: From 2012 to 2014, 134 from 249 (54%) consecutive male outpatients reported erectile dysfunction (ED), 37% of them related to opioids and 19% evidenced OPIAD. A total of 120 subjects (94 cases and 26 matched-controls) were included. A significantly lower luteinizing hormone, Total-T and Free-T were found, as well as, a significant correlation between Sal-T and Total-T (r = 0.234, p = 0.039), Bio-T (r = 0.241, p = 0.039), IIEF (r = 0.363, p = 0.003) and HAD-anxiety (r = -0.414, p = 0.012) in OPIAD patients. Sal-T levels were significantly lower in patients with severe-moderate ED versus mild ED (p = 0.045) and in patients with severe ED versus moderate-mild ED (p = 0.036). CONCLUSIONS: These data demonstrate the high prevalence of ED in long-term use of opioids, part of this is associated to OPIAD, which can be tested by Sal-T as a non-invasive approach.
[Study of the month : FLAME study in chronic obstructive pulmonary disease].[Pubmed:28383836]
Rev Med Liege. 2016 Sep;71(9):400-406.
The place of combinations of bronchodilators (longacting beta-agonist / muscarinic agonist or LABA / LAMA) in the prevention of the exacerbations of the chronic obstructive pulmonary disease (COPD) is not still clearly established, and need a comparison with combination of LABA/ inhaled steroids. FLAME was a randomized non-inferiority phase 3 study comparing indacaterol/glycopyrronium 110/50 mug (IND/GLY) once daily with salmeterol/proprionate of fluticasone 50/500 mug (SAL/FC) twice daily. The primary objective of the study was to demonstrate that IND/GLY was non-inferior to SAL/FC in terms of reduction of all COPD exacerbations (mild/moderate/severe) during 52 weeks of treatment in patients having had at least 1 exacerbation in previous 12 months. The combination IND/GLY showed not only non inferiority, but also superiority, to SAL/FC in reducing the annual rate of all COPD exacerbations (mild, moderate, or severe) by 11 % by comparison with SAL/FC (p = 0.003), and by 17 % for the annual rate of moderate or severe exacerbations (p inferior to 0.001). IND/GLY prolonged the time to the first COPD exacerbation by 16 % for mild, 22 % for moderate and 19 % for severe by comparison with SAL/FC (all with p inferior to 0.05). FLAME study showed that IND/GLY, a steroid-free option, is more effective than SAL/FC in preventing COPD exacerbations in patients with one or more exacerbations in the past year.
The impact of diet and arginine supplementation on pancreatic mass, digestive enzyme activity, and insulin-containing cell cluster morphology during the estrous cycle in sheep.[Pubmed:27875754]
Domest Anim Endocrinol. 2017 Apr;59:23-29.
To determine the effect of feed intake and arginine treatment during different stages of the estrous cycle on pancreatic mass, digestive enzyme activity, and histological measurements, ewes (n = 120) were randomly allocated to 1 of 3 dietary groups; control (CON; 2.14-Mcal metabolizable energy/kg), underfed (UF; 0.6 x CON), or overfed (OF; 2 x CON) over 2 yr. Estrus was synchronized using a controlled internal drug release device for 14 d. At controlled internal drug release withdrawal, ewes from each dietary group were assigned to 1 of 2 treatments; Arg (L-Arg HCl, 155-mumol/kg BW) or Sal (approximately 10-mL saline). Treatments were administered 3 times daily via jugular catheter and continued until slaughter on d (day) 5 and 10 of the second estrus cycle (early luteal phase, n = 41 and mid-luteal phase, n = 39; yr 1) and d 15 of the first estrus cycle (late luteal phase, n = 40; yr 2). A blood sample collected from jugular catheters for serum insulin analysis before slaughter. The pancreas was then removed, trimmed of mesentery and fat, weighed, and a sample snap-frozen until enzyme analysis. Additional pancreatic samples were fixed in 10% formalin solution for histological examination of size and distribution of insulin-containing cell clusters. Data were analyzed as a completely randomized design with a factorial arrangement of treatments. Diet, treatment, and diet x treatment were blocked by yr and included in the model with initial BW used as a covariate. Day of the estrous cycle was initially included in the model but later removed as no effects (P > 0.10) were observed for any pancreatic variables tested. Overfed ewes had the greatest (P < 0.001) change in BW, final BW, change in BCS, and final BCS. A diet x treatment interaction was observed for change in BW and final BW (P
PKR and PKR-like endoplasmic reticulum kinase induce the proteasome-dependent degradation of cyclin D1 via a mechanism requiring eukaryotic initiation factor 2alpha phosphorylation.[Pubmed:18063576]
J Biol Chem. 2008 Feb 8;283(6):3097-108.
Cyclin D1 plays a critical role in controlling the G(1)/S transition via the regulation of cyclin-dependent kinase activity. Several studies have indicated that cyclin D1 translation is decreased upon activation of the eukaryotic initiation factor 2alpha (eIF2alpha) kinases. We examined the effect of activation of the eIF2alpha kinases PKR and PKR-like endoplasmic reticulum kinase (PERK) on cyclin D1 protein levels and translation and determined that cyclin D1 protein levels decrease upon the induction of PKR and PERK catalytic activity but that this decrease is not due to translation. Inhibition of the 26 S proteasome with MG132 rescued cyclin D1 protein levels, indicating that rather than inhibiting translation, PKR and PERK act to increase cyclin D1 degradation. Interestingly, this effect still requires eIF2alpha phosphorylation at serine 51, as cyclin D1 remains unaffected in cells containing a non-phosphorylatable form of the protein. This proteasome-dependent degradation of cyclin D1 requires an intact ubiquitination pathway, although the ubiquitination of cyclin D1 is not itself affected. Furthermore, this degradation is independent of phosphorylation of cyclin D1 at threonine 286, which is mediated by the glycogen synthase kinase 3beta and mitogen-activated protein kinase pathways as described in previous studies. Our study reveals a novel functional cross-talk between eIF2alpha phosphorylation and the proteasomal degradation of cyclin D1 and that this degradation is dependent upon eIF2alpha phosphorylation during short, but not prolonged, periods of stress.
The eIF2alpha kinases PERK and PKR activate glycogen synthase kinase 3 to promote the proteasomal degradation of p53.[Pubmed:17785458]
J Biol Chem. 2007 Oct 26;282(43):31675-87.
Phosphorylation of eukaryotic initiation factor 2alpha (eIF2alpha) is mediated by a family of kinases that respond to various forms of environmental stress. The eIF2alpha kinases are critical for mRNA translation, cell proliferation, and apoptosis. Activation of the tumor suppressor p53 results in cell cycle arrest and apoptosis in response to various types of stress. We previously showed that, unlike the majority of stress responses that stabilize and activate p53, induction of endoplasmic reticulum stress leads to p53 degradation through an Mdm2-dependent mechanism. Here, we demonstrate that the endoplasmic reticulum-resident eIF2alpha kinase PERK mediates the proteasomal degradation of p53 independently of translational control. This role is not specific for PERK, because the eIF2alpha kinase PKR also promotes p53 degradation in response to double-stranded RNA. We further establish that the eIF2alpha kinases induce glycogen synthase kinase 3 to promote the nuclear export and proteasomal degradation of p53. Our findings reveal a novel cross-talk between the eIF2alpha kinases and p53 with implications in cell proliferation and tumorigenesis.
eIF2alpha phosphorylation bidirectionally regulates the switch from short- to long-term synaptic plasticity and memory.[Pubmed:17418795]
Cell. 2007 Apr 6;129(1):195-206.
The late phase of long-term potentiation (LTP) and memory (LTM) requires new gene expression, but the molecular mechanisms that underlie these processes are not fully understood. Phosphorylation of eIF2alpha inhibits general translation but selectively stimulates translation of ATF4, a repressor of CREB-mediated late-LTP (L-LTP) and LTM. We used a pharmacogenetic bidirectional approach to examine the role of eIF2alpha phosphorylation in synaptic plasticity and behavioral learning. We show that in eIF2alpha(+/S51A) mice, in which eIF2alpha phosphorylation is reduced, the threshold for eliciting L-LTP in hippocampal slices is lowered, and memory is enhanced. In contrast, only early-LTP is evoked by repeated tetanic stimulation and LTM is impaired, when eIF2alpha phosphorylation is increased by injecting into the hippocampus a small molecule, Sal003, which prevents the dephosphorylation of eIF2alpha. These findings highlight the importance of a single phosphorylation site in eIF2alpha as a key regulator of L-LTP and LTM formation.


