Taccalonolide AJCAS# 1349904-82-0 |
Quality Control & MSDS
Number of papers citing our products

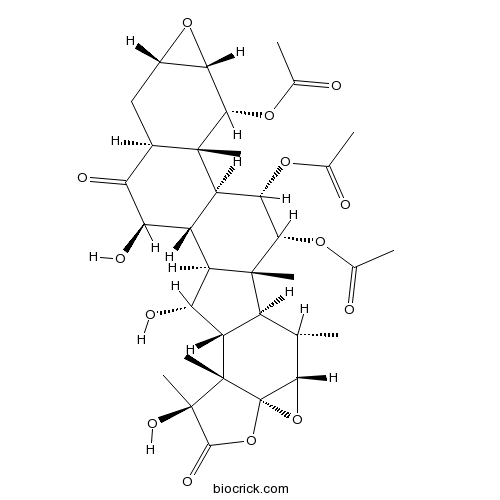
Chemical structure

3D structure
| Cas No. | 1349904-82-0 | SDF | Download SDF |
| PubChem ID | 56926890 | Appearance | Powder |
| Formula | C34H44O14 | M.Wt | 676.7 |
| Type of Compound | Steroids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| SMILES | CC1C2C(C(C3C2(C(C(C4C3C(C(=O)C5C4(C(C6C(C5)O6)OC(=O)C)C)O)OC(=O)C)OC(=O)C)C)O)C7(C(C(=O)OC78C1O8)(C)O)C | ||
| Standard InChIKey | BWKYBGRKQMTOQL-MPOFNYKTSA-N | ||
| Standard InChI | InChI=1S/C34H44O14/c1-10-17-20(32(7)33(8,42)29(41)48-34(32)26(10)47-34)23(40)18-16-19(25(43-11(2)35)28(31(17,18)6)45-13(4)37)30(5)14(21(38)22(16)39)9-15-24(46-15)27(30)44-12(3)36/h10,14-20,22-28,39-40,42H,9H2,1-8H3/t10-,14+,15-,16-,17-,18+,19+,20-,22+,23+,24-,25-,26-,27-,28-,30-,31+,32-,33+,34+/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Taccalonolide AJ is a microtubule stabilizer; it has excellent and highly persistent antitumor efficacy when administered directly to the tumor, suggesting that the lack of antitumor efficacy seen with systemic administration of AJ is likely due to its short half-life in vivo. |
| In vivo | Pharmacokinetic Analysis and in Vivo Antitumor Efficacy of Taccalonolides AF and AJ.[Pubmed: 28112516 ]J Nat Prod. 2017 Feb 24;80(2):409-414.The taccalonolides are microtubule stabilizers that covalently bind tubulin and circumvent clinically relevant forms of resistance to other drugs of this class. Efforts are under way to identify a taccalonolide with optimal properties for clinical development. The structurally similar taccalonolides AF and AJ have comparable microtubule-stabilizing activities in vitro, but taccalonolide AF has excellent in vivo antitumor efficacy when administered systemically, while Taccalonolide AJ does not elicit this activity even at maximum tolerated dose. |
| Kinase Assay | Chemically diverse microtubule stabilizing agents initiate distinct mitotic defects and dysregulated expression of key mitotic kinases.[Pubmed: 23399639]Biochem Pharmacol. 2013 Apr 15;85(8):1104-14.
|

Taccalonolide AJ Dilution Calculator

Taccalonolide AJ Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.4778 mL | 7.3888 mL | 14.7776 mL | 29.5552 mL | 36.944 mL |
| 5 mM | 0.2956 mL | 1.4778 mL | 2.9555 mL | 5.911 mL | 7.3888 mL |
| 10 mM | 0.1478 mL | 0.7389 mL | 1.4778 mL | 2.9555 mL | 3.6944 mL |
| 50 mM | 0.0296 mL | 0.1478 mL | 0.2956 mL | 0.5911 mL | 0.7389 mL |
| 100 mM | 0.0148 mL | 0.0739 mL | 0.1478 mL | 0.2956 mL | 0.3694 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- SAR245409
Catalog No.:BCC2534
CAS No.:934493-76-2
- 4-P-PDOT
Catalog No.:BCC6900
CAS No.:134865-74-0
- 8-M-PDOT
Catalog No.:BCC6901
CAS No.:134865-70-6
- 6''-O-Acetylglycitin
Catalog No.:BCN3866
CAS No.:134859-96-4
- A 412997 dihydrochloride
Catalog No.:BCC6224
CAS No.:1347744-96-0
- Linderane
Catalog No.:BCN5023
CAS No.:13476-25-0
- Lamivudine
Catalog No.:BCC3801
CAS No.:134678-17-4
- GSK343
Catalog No.:BCC1607
CAS No.:1346704-33-3
- GSK621
Catalog No.:BCC6517
CAS No.:1346607-05-3
- GSK126
Catalog No.:BCC1604
CAS No.:1346574-57-9
- GSK503
Catalog No.:BCC6386
CAS No.:1346572-63-1
- ML 240
Catalog No.:BCC5604
CAS No.:1346527-98-7
- 2,2'-Dithiobisbenzanilide
Catalog No.:BCC8497
CAS No.:135-57-9
- Pseudotropine
Catalog No.:BCN1932
CAS No.:135-97-7
- Z-Hyp-OH
Catalog No.:BCC3257
CAS No.:13504-85-3
- Clopidogrel bisulfate
Catalog No.:BCC8917
CAS No.:135046-48-9
- MK-5172 hydrate
Catalog No.:BCC1763
CAS No.:1350462-55-3
- MK-5172
Catalog No.:BCC1762
CAS No.:1350514-68-9
- Repaglinide
Catalog No.:BCC2504
CAS No.:135062-02-1
- kb-NB77-78
Catalog No.:BCC5462
CAS No.:1350622-33-1
- Camelliaside A
Catalog No.:BCN3871
CAS No.:135095-52-2
- Fmoc-Nva-OH
Catalog No.:BCC3302
CAS No.:135112-28-6
- Z-Tyr-OMe
Catalog No.:BCC2746
CAS No.:13512-31-7
- 2',7-Dihydroxy-5,8-dimethoxyflavanone
Catalog No.:BCN4041
CAS No.:1351338-14-1
Chemically diverse microtubule stabilizing agents initiate distinct mitotic defects and dysregulated expression of key mitotic kinases.[Pubmed:23399639]
Biochem Pharmacol. 2013 Apr 15;85(8):1104-14.
Microtubule stabilizers are some of the most successful drugs used in the treatment of adult solid tumors and yet the molecular events responsible for their antimitotic actions are not well defined. The mitotic events initiated by three structurally and biologically diverse microtubule stabilizers; Taccalonolide AJ, laulimalide/fijianolide B and paclitaxel were studied. These microtubule stabilizers cause the formation of aberrant, but structurally distinct mitotic spindles leading to the hypothesis that they differentially affect mitotic signaling. Each microtubule stabilizer initiated different patterns of expression of key mitotic signaling proteins. Taccalonolide AJ causes centrosome separation and disjunction failure to a much greater extent than paclitaxel or laulimalide, which is consistent with the distinct defects in expression and activation of Plk1 and Eg5 caused by each stabilizer. Localization studies revealed that TPX2 and Aurora A are associated with each spindle aster formed by each stabilizer. This suggests a common mechanism of aster formation. However, Taccalonolide AJ also causes pericentrin accumulation on every spindle aster. The presence of pericentrin at every spindle aster initiated by Taccalonolide AJ might facilitate the maintenance and stability of the highly focused asters formed by this stabilizer. Laulimalide and paclitaxel cause completely different patterns of expression and activation of these proteins, as well as phenotypically different spindle phenotypes. Delineating how diverse microtubule stabilizers affect mitotic signaling pathways could identify key proteins involved in modulating sensitivity and resistance to the antimitotic actions of these compounds.
Pharmacokinetic Analysis and in Vivo Antitumor Efficacy of Taccalonolides AF and AJ.[Pubmed:28112516]
J Nat Prod. 2017 Feb 24;80(2):409-414.
The taccalonolides are microtubule stabilizers that covalently bind tubulin and circumvent clinically relevant forms of resistance to other drugs of this class. Efforts are under way to identify a taccalonolide with optimal properties for clinical development. The structurally similar taccalonolides AF and AJ have comparable microtubule-stabilizing activities in vitro, but taccalonolide AF has excellent in vivo antitumor efficacy when administered systemically, while Taccalonolide AJ does not elicit this activity even at maximum tolerated dose. The hypothesis that pharmacokinetic differences underlie the differential efficacies of taccalonolides AF and AJ was tested. The effects of serum on their in vivo potency, metabolism by human liver microsomes and in vivo pharmacokinetic properties were evaluated. Taccalonolides AF and AJ were found to have elimination half-lives of 44 and 8.1 min, respectively. Furthermore, Taccalonolide AJ was found to have excellent and highly persistent antitumor efficacy when administered directly to the tumor, suggesting that the lack of antitumor efficacy seen with systemic administration of AJ is likely due to its short half-life in vivo. These results help define why some, but not all, taccalonolides inhibit the growth of tumors at systemically tolerable doses and prompt studies to further improve their pharmacokinetic profile and antitumor efficacy.


