ThapsigarginPotent inhibitor of SERCA ATPase CAS# 67526-95-8 |
- Lenalidomide hydrochloride
Catalog No.:BCC1697
CAS No.:1243329-97-6
- Celastrol
Catalog No.:BCN5986
CAS No.:34157-83-0
- Necrostatin 2 racemate
Catalog No.:BCC2077
CAS No.:852391-15-2
- Necrostatin 2
Catalog No.:BCC1793
CAS No.:852391-19-6
- Necrostatin 2 S enantiomer
Catalog No.:BCC2078
CAS No.:852391-20-9
Quality Control & MSDS
Number of papers citing our products

Chemical structure

3D structure
| Cas No. | 67526-95-8 | SDF | Download SDF |
| PubChem ID | 446378 | Appearance | Powder |
| Formula | C34H50O12 | M.Wt | 650.76 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : 250 mg/mL (384.17 mM; Need ultrasonic) | ||
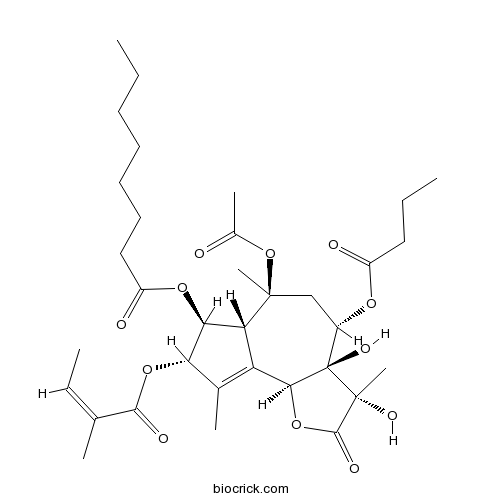
| Chemical Name | [(3S,3aR,4S,6S,6aR,7S,8S,9bS)-6-acetyloxy-4-butanoyloxy-3,3a-dihydroxy-3,6,9-trimethyl-8-[(Z)-2-methylbut-2-enoyl]oxy-2-oxo-4,5,6a,7,8,9b-hexahydroazuleno[4,5-b]furan-7-yl] octanoate | ||
| SMILES | CCCCCCCC(=O)OC1C2C(=C(C1OC(=O)C(=CC)C)C)C3C(C(CC2(C)OC(=O)C)OC(=O)CCC)(C(C(=O)O3)(C)O)O | ||
| Standard InChIKey | IXFPJGBNCFXKPI-FSIHEZPISA-N | ||
| Standard InChI | InChI=1S/C34H50O12/c1-9-12-13-14-15-17-24(37)43-28-26-25(20(5)27(28)44-30(38)19(4)11-3)29-34(41,33(8,40)31(39)45-29)22(42-23(36)16-10-2)18-32(26,7)46-21(6)35/h11,22,26-29,40-41H,9-10,12-18H2,1-8H3/b19-11-/t22-,26+,27-,28-,29-,32-,33+,34+/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Potent inhibitor of sarco-endoplasmic reticulum Ca2+-ATPases. Causes ER stress; can be used to induce autophagy in mammalian cells. |

Thapsigargin Dilution Calculator

Thapsigargin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.5367 mL | 7.6833 mL | 15.3666 mL | 30.7333 mL | 38.4166 mL |
| 5 mM | 0.3073 mL | 1.5367 mL | 3.0733 mL | 6.1467 mL | 7.6833 mL |
| 10 mM | 0.1537 mL | 0.7683 mL | 1.5367 mL | 3.0733 mL | 3.8417 mL |
| 50 mM | 0.0307 mL | 0.1537 mL | 0.3073 mL | 0.6147 mL | 0.7683 mL |
| 100 mM | 0.0154 mL | 0.0768 mL | 0.1537 mL | 0.3073 mL | 0.3842 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 4-Methoxy-5-(3-morpholinopropoxy)-2-nitrobenzonitrile
Catalog No.:BCC8710
CAS No.:675126-26-8
- Spathulenol
Catalog No.:BCN4227
CAS No.:6750-60-3
- Arnidiol
Catalog No.:BCN3810
CAS No.:6750-30-7
- Eupatolide
Catalog No.:BCN7345
CAS No.:6750-25-0
- Fuegin
Catalog No.:BCN5809
CAS No.:6750-10-3
- DIDS
Catalog No.:BCC7942
CAS No.:67483-13-0
- GBR 12935 dihydrochloride
Catalog No.:BCC5380
CAS No.:67469-81-2
- Vanoxerine dihydrochloride
Catalog No.:BCC5129
CAS No.:67469-78-7
- GBR 12783 dihydrochloride
Catalog No.:BCC6676
CAS No.:67469-75-4
- Vanoxerine
Catalog No.:BCC5130
CAS No.:67469-69-6
- GBR 13069 dihydrochloride
Catalog No.:BCC6640
CAS No.:67469-45-8
- Amorolfine
Catalog No.:BCC8819
CAS No.:67467-83-8
- Alpha-caryophyllene
Catalog No.:BCN3877
CAS No.:6753-98-6
- Helenalin
Catalog No.:BCN8073
CAS No.:6754-13-8
- Dehydrotumulosic acid
Catalog No.:BCN3740
CAS No.:6754-16-1
- Polygodial
Catalog No.:BCC7597
CAS No.:6754-20-7
- 8'-Epicleomiscosin A
Catalog No.:BCC3917
CAS No.:
- Nutlin-3b
Catalog No.:BCC1156
CAS No.:675576-97-3
- Nutlin-3a chiral
Catalog No.:BCC1812
CAS No.:675576-98-4
- PG 01037 dihydrochloride
Catalog No.:BCC7801
CAS No.:675599-62-9
- Lariciresinol dimethyl ether
Catalog No.:BCN4228
CAS No.:67560-68-3
- Maoyecrystal E
Catalog No.:BCN3283
CAS No.:675603-39-1
- Baicalein 7-O-beta-D-ethylglucuronide
Catalog No.:BCN7981
CAS No.:675624-38-1
- Diosbulbin G
Catalog No.:BCN4229
CAS No.:67567-15-1
Calpain mobilizes Atg9/Bif-1 vesicles from Golgi stacks upon autophagy induction by thapsigargin.[Pubmed:28302665]
Biol Open. 2017 May 15;6(5):551-562.
CAPNS1 is essential for stability and function of the ubiquitous calcium-dependent proteases micro- and milli-calpain. Upon inhibition of the endoplasmic reticulum Ca(2+) ATPase by 100 nM Thapsigargin, both micro-calpain and autophagy are activated in human U2OS osteosarcoma cells in a CAPNS1-dependent manner. As reported for other autophagy triggers, Thapsigargin treatment induces Golgi fragmentation and fusion of Atg9/Bif-1-containing vesicles with LC3 bodies in control cells. By contrast, CAPNS1 depletion is coupled with an accumulation of LC3 bodies and Rab5 early endosomes. Moreover, Atg9 and Bif-1 remain in the GM130-positive Golgi stacks and Atg9 fails to interact with the endocytic route marker transferrin receptor and with the core autophagic protein Vps34 in CAPNS1-depleted cells. Ectopic expression of a Bif-1 point mutant resistant to calpain processing is coupled to endogenous p62 and LC3-II accumulation. Altogether, these data indicate that calpain allows dynamic flux of Atg9/Bif-1 vesicles from the Golgi toward the budding autophagosome.
Structure/activity relationship of thapsigargin inhibition on the purified Golgi/secretory pathway Ca(2+)/Mn(2+)-transport ATPase (SPCA1a).[Pubmed:28264934]
J Biol Chem. 2017 Apr 28;292(17):6938-6951.
The Golgi/secretory pathway Ca(2+)/Mn(2+)-transport ATPase (SPCA1a) is implicated in breast cancer and Hailey-Hailey disease. Here, we purified recombinant human SPCA1a from Saccharomyces cerevisiae and measured Ca(2+)-dependent ATPase activity following reconstitution in proteoliposomes. The purified SPCA1a displays a higher apparent Ca(2+) affinity and a lower maximal turnover rate than the purified sarco(endo)plasmic reticulum Ca(2+)-ATPase (SERCA1a). The lipids cholesteryl hemisuccinate, linoleamide/oleamide, and phosphatidylethanolamine inhibit and phosphatidic acid and sphingomyelin enhance SPCA1a activity. Moreover, SPCA1a is blocked by micromolar concentrations of the commonly used SERCA1a inhibitors Thapsigargin (Tg), cyclopiazonic acid, and 2,5-di-tert-butylhydroquinone. Because tissue-specific targeting of SERCA2b by Tg analogues is considered for prostate cancer therapy, the inhibition of SPCA1a by Tg might represent an off-target risk. We assessed the structure-activity relationship (SAR) of Tg for SPCA1a by in silico modeling, site-directed mutagenesis, and measuring the potency of a series of Tg analogues. These indicate that Tg and the analogues are bound via the Tg scaffold but with lower affinity to the same homologous cavity as on the membrane surface of SERCA1a. The lower Tg affinity may depend on a more flexible binding cavity in SPCA1a, with low contributions of the Tg O-3, O-8, and O-10 chains to the binding energy. Conversely, the protein interaction of the Tg O-2 side chain with SPCA1a appears comparable with that of SERCA1a. These differences define a SAR of Tg for SPCA1a distinct from that of SERCA1a, indicating that Tg analogues with a higher specificity for SPCA1a can probably be developed.
Localization and in-Vivo Characterization of Thapsia garganica CYP76AE2 Indicates a Role in Thapsigargin Biosynthesis.[Pubmed:28275147]
Plant Physiol. 2017 May;174(1):56-72.
The Mediterranean plant Thapsia garganica (dicot, Apiaceae), also known as deadly carrot, produces the highly toxic compound Thapsigargin. This compound is a potent inhibitor of the sarcoplasmic-endoplasmic reticulum Ca(2+)-ATPase calcium pump in mammals and is of industrial importance as the active moiety of the anticancer drug mipsagargin, currently in clinical trials. Knowledge of Thapsigargin in planta storage and biosynthesis has been limited. Here, we present the putative second step in Thapsigargin biosynthesis, by showing that the cytochrome P450 TgCYP76AE2, transiently expressed in Nicotiana benthamiana, converts epikunzeaol into epidihydrocostunolide. Furthermore, we show that Thapsigargin is likely to be stored in secretory ducts in the roots. Transcripts from TgTPS2 (epikunzeaol synthase) and TgCYP76AE2 in roots were found only in the epithelial cells lining these secretory ducts. This emphasizes the involvement of these cells in the biosynthesis of Thapsigargin. This study paves the way for further studies of Thapsigargin biosynthesis.
The endoplasmic reticulum stress inducer thapsigargin enhances the toxicity of ZnO nanoparticles to macrophages and macrophage-endothelial co-culture.[Pubmed:28171821]
Environ Toxicol Pharmacol. 2017 Mar;50:103-110.
It was recently shown that exposure to ZnO nanoparticles (NPs) could induce endoplasmic reticulum (ER) stress both in vivo and in vitro, but the role of ER stress in ZnO NP induced toxicity remains unclear. Because macrophages are sensitive to ER stress, we hypothesized that stressing macrophages with ER stress inducer could enhance the toxicity of ZnO NPs. In this study, the effects of ER stress inducer Thapsigargin (TG) on the toxicity of ZnO NPs to THP-1 macrophages were investigated. The results showed that TG enhanced ZnO NP induced cytotoxicity as revealed by water soluble tetrazolium-1 (WST-1) and neutral red uptake assays, but not lactate dehydrogenase (LDH) assay. ZnO NPs dose-dependently enhanced the accumulation of intracellular Zn ions without the induction of reactive oxygen species (ROS), and the presence of TG did not significantly affect these effects. In the co-culture, exposure of THP-1 macrophages in the upper chamber to ZnO NPs and TG significantly reduced the viability of human umbilical vein endothelial cells (HUVECs) in the lower chamber, but the release of tumor necrosis factor alpha (TNFalpha) was not induced. In summary, our data showed that stressing THP-1 macrophages with TG enhanced the cytotoxicity of ZnO NPs to macrophages and macrophage-endothelial co-cultures.
Differential effects of endoplasmic reticulum stress-induced autophagy on cell survival.[Pubmed:17135238]
J Biol Chem. 2007 Feb 16;282(7):4702-10.
Autophagy is a cellular response to adverse environment and stress, but its significance in cell survival is not always clear. Here we show that autophagy could be induced in the mammalian cells by chemicals, such as A23187, tunicamycin, Thapsigargin, and brefeldin A, that cause endoplasmic reticulum stress. Endoplasmic reticulum stress-induced autophagy is important for clearing polyubiquitinated protein aggregates and for reducing cellular vacuolization in HCT116 colon cancer cells and DU145 prostate cancer cells, thus mitigating endoplasmic reticulum stress and protecting against cell death. In contrast, autophagy induced by the same chemicals does not confer protection in a normal human colon cell line and in the non-transformed murine embryonic fibroblasts but rather contributes to cell death. Thus the impact of autophagy on cell survival during endoplasmic reticulum stress is likely contingent on the status of cells, which could be explored for tumor-specific therapy.
A tool coming of age: thapsigargin as an inhibitor of sarco-endoplasmic reticulum Ca(2+)-ATPases.[Pubmed:9612087]
Trends Pharmacol Sci. 1998 Apr;19(4):131-5.
Thapsigargin is the most widely used inhibitor of the ubiquitous sarco-endoplasmic reticulum Ca(2+)-ATPases in mammalian cells. Over the past ten years, this guaianolide compound of plant origin has become a popular tool in a host of studies directed at elucidating the mechanisms of intracellular Ca2+ signalling. Its remarkable potency and selectivity have been instrumental in widening our view of the function of intracellular Ca2+ stores to include such key aspects as store-operated Ca2+ entry or the involvement of the stores in protein synthesis or cell growth. In this article Marek Treiman, Casper Caspersen and Soren Brogger Christensen review the key pharmacological features of Thapsigargin action; they also discuss some of the ways in which its unique properties have shown to be important for obtaining new insights into the biology of Ca2+ stores, and how these properties might encompass a therapeutic potential. In parallel, attention is drawn to some of the limitations and pitfalls encountered when working with Thapsigargin.
Specific substitutions at amino acid 256 of the sarcoplasmic/endoplasmic reticulum Ca2+ transport ATPase mediate resistance to thapsigargin in thapsigargin-resistant hamster cells.[Pubmed:9452480]
J Biol Chem. 1998 Feb 6;273(6):3542-6.
High levels of resistance to Thapsigargin (TG), a specific inhibitor of intracellular Ca2+ transport ATPases (SERCAs), can be developed in culture by stepwise exposure of mammalian cells to increasing concentrations of TG. We have identified, in two independently selected TG-resistant hamster cell lines of different lineages, mutant forms of SERCA. In the TG-resistant Chinese hamster lung fibroblast cell line DC-3F/TG, a T --> C change at nucleotide 766 introduces a Phe256 --> Leu alteration within the first cytosolic loop of the SERCA. In contrast, in the TG-resistant Syrian hamster smooth muscle cell line DDT/TG 4 microM, a T --> C change at nucleotide 767 introduces a Phe256 --> Ser mutation at that position. When these specific mutations are introduced into a wild-type full-length avian SERCA1 cDNA, transfection experiments reveal that Ca2+ transport function and ATP hydrolytic activity are not altered by such mutations. However, a 4-5-fold resistance to TG inhibition of Ca2+ transport function occurs upon the introduction of either the Phe256 --> Leu or the Phe256 --> Ser mutation into wild-type SERCA1. These specific mutations also render the hydrolytic activity of the ATPase resistant to inhibition by TG. Our results not only implicate amino acid 256 in TG-SERCA interactions, but also demonstrate that specific mutations within SERCA can mediate resistance to TG.
Kinetics of thapsigargin-Ca(2+)-ATPase (sarcoplasmic reticulum) interaction reveals a two-step binding mechanism and picomolar inhibition.[Pubmed:7744817]
J Biol Chem. 1995 May 19;270(20):11731-4.
Thapsigargin is a high affinity inhibitor of sarco- and endoplasmic reticulum (SERCA) type ATPases. We have used kinetics to determine the dissociation constant of Thapsigargin-sarcoplasmic reticulum Ca(2+)-ATPase interaction in the absence and presence of non-ionic detergent. The observed "off" rate constant was measured as 0.0052 s-1 at 26 degrees C by the kinetics of inhibition of ATPase activity following transfer from an inactivated Thapsigargin-ATPase complex to native ATPase. Inactive ATPase was produced by cross-linking the active site with glutaraldehyde. The observed dissociation rate constant was increased 7-fold by 0.1% Triton X-100, indicating that perturbation of the transmembrane and stalk region by detergent altered the binding parameters of the inhibitor. In addition, Thapsigargin stabilized the ATPase against inactivation caused by detergent in the absence of Ca2+. The observed "on" rate constant of Thapsigargin was measured at 26 degrees C as 25 s-1 irrespective of Thapsigargin concentration, by the kinetics of Thapsigargin- induced change in intrinsic fluorescence. An Arrhenius plot showed a temperature dependence of this rate constant, indicative of a conformational change in the protein with an activation energy of 9.5 kcal/mol for Thapsigargin binding. The affinity of the Ca(2+)-ATPase for Thapsigargin was calculated to be greater than 2 pM at pH 7.0 and 26 degrees C.


