VanoxerineDAT1 antagonist CAS# 67469-69-6 |
- Quetiapine fumarate
Catalog No.:BCN5339
CAS No.:111974-72-2
- PD128907 HCl
Catalog No.:BCC4469
CAS No.:112960-16-4
Quality Control & MSDS
Number of papers citing our products

Chemical structure

3D structure
| Cas No. | 67469-69-6 | SDF | Download SDF |
| PubChem ID | 3455 | Appearance | Powder |
| Formula | C28H32F2N2O | M.Wt | 450.56 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | GBR 12909; I893 | ||
| Solubility | Soluble in DMSO | ||
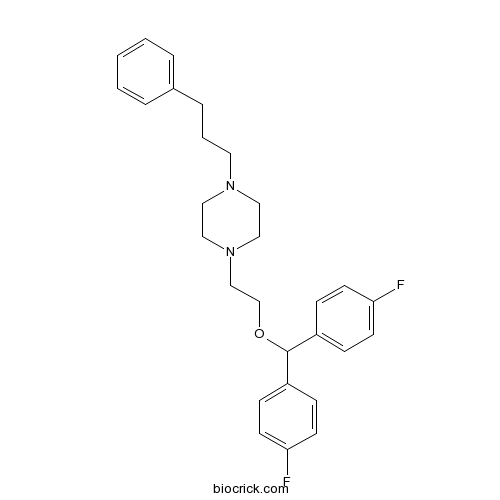
| Chemical Name | 1-[2-[bis(4-fluorophenyl)methoxy]ethyl]-4-(3-phenylpropyl)piperazine | ||
| SMILES | C1CN(CCN1CCCC2=CC=CC=C2)CCOC(C3=CC=C(C=C3)F)C4=CC=C(C=C4)F | ||
| Standard InChIKey | NAUWTFJOPJWYOT-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C28H32F2N2O/c29-26-12-8-24(9-13-26)28(25-10-14-27(30)15-11-25)33-22-21-32-19-17-31(18-20-32)16-4-7-23-5-2-1-3-6-23/h1-3,5-6,8-15,28H,4,7,16-22H2 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Vanoxerine (GBR12909) is a potent and selective DRI (Dopamine reuptake inhibitor).
IC50 value:
Target: Dopamine reuptake inhibitor
At a cellular level, vanoxerine acts to block cardiac ion channels. Vanoxerine is a multichannel blocker, acting on IKr (potassium), L-type calcium and sodium ion channels.[14] By blocking these specific channels, there is a prolongation of the action potential of the cell, preventing reactivation by a reentrant circuit. The block is strongly frequency dependant: as the pacing of the heart increases so does the frequency of ion channel blocking by vanoxerine. Vanoxerine is a potentially effective treatment for cardiac arrhythmias. A significant cause of cardiac arrhythmias is reentry, an electrophysiologic event in which the proliferating signal that refuses to terminate, and endures to reexcite the heart after the refractory period. References: | |||||

Vanoxerine Dilution Calculator

Vanoxerine Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.2195 mL | 11.0973 mL | 22.1946 mL | 44.3892 mL | 55.4865 mL |
| 5 mM | 0.4439 mL | 2.2195 mL | 4.4389 mL | 8.8778 mL | 11.0973 mL |
| 10 mM | 0.2219 mL | 1.1097 mL | 2.2195 mL | 4.4389 mL | 5.5487 mL |
| 50 mM | 0.0444 mL | 0.2219 mL | 0.4439 mL | 0.8878 mL | 1.1097 mL |
| 100 mM | 0.0222 mL | 0.111 mL | 0.2219 mL | 0.4439 mL | 0.5549 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Vanoxerine is an antagonist of dopamine transporter (DAT1) with Ki value of 16.9nM [1].
As an antagonist of DAT, vanoxerine is developed for treatment of Parkinson's disease and depression but has no effect on these diseases. Vanoxerine is also found to have desirable cardiac antiarrhythmic properties. It is a blocker of cardiac hERG (hKv11.1) with IC50 value of 0.84nM. It also blocks the ICa,L and hNav1.5 channel with IC50 values of 320nM and 830nM, respectively. Vanoxerine does not significantly prolong Purkinje fiber APD60 and APD90 and has no significant effect on QT or TDR. Further, the clinical trial demonstrates that the effective concentrations of vanoxerine are well tolerated and safe in man [2].
References:
[1] Giros B, el Mestikawy S, Godinot N, Zheng K, Han H, Yang-Feng T, Caron MG. Cloning, pharmacological characterization, and chromosome assignment of the human dopamine transporter. Mol Pharmacol. 1992 Sep;42(3):383-90.
[2] Lacerda AE, Kuryshev YA, Yan GX, Waldo AL, Brown AM. Vanoxerine: cellular mechanism of a new antiarrhythmic. J Cardiovasc Electrophysiol. 2010 Mar;21(3):301-10.
- GBR 13069 dihydrochloride
Catalog No.:BCC6640
CAS No.:67469-45-8
- Amorolfine
Catalog No.:BCC8819
CAS No.:67467-83-8
- Isoasatone A
Catalog No.:BCN7762
CAS No.:67451-73-4
- Fmoc-Cys(tBu)-OH
Catalog No.:BCC3478
CAS No.:67436-13-9
- Isorhamnetin-3-O-galactoside
Catalog No.:BCC8190
CAS No.:6743-92-6
- (±)-Blebbistatin
Catalog No.:BCC7169
CAS No.:674289-55-5
- Z-D-His-OH
Catalog No.:BCC2767
CAS No.:67424-93-5
- 3-O-Acetyl-11-keto-beta-boswellic acid
Catalog No.:BCN1381
CAS No.:67416-61-9
- H-D-Lys-OMe.2HCl
Catalog No.:BCC2680
CAS No.:67396-08-1
- Drospirenone
Catalog No.:BCC4493
CAS No.:67392-87-4
- Catalposide
Catalog No.:BCN4225
CAS No.:6736-85-2
- Pterokaurane R
Catalog No.:BCN4076
CAS No.:67349-43-3
- GBR 12783 dihydrochloride
Catalog No.:BCC6676
CAS No.:67469-75-4
- Vanoxerine dihydrochloride
Catalog No.:BCC5129
CAS No.:67469-78-7
- GBR 12935 dihydrochloride
Catalog No.:BCC5380
CAS No.:67469-81-2
- DIDS
Catalog No.:BCC7942
CAS No.:67483-13-0
- Fuegin
Catalog No.:BCN5809
CAS No.:6750-10-3
- Eupatolide
Catalog No.:BCN7345
CAS No.:6750-25-0
- Arnidiol
Catalog No.:BCN3810
CAS No.:6750-30-7
- Spathulenol
Catalog No.:BCN4227
CAS No.:6750-60-3
- 4-Methoxy-5-(3-morpholinopropoxy)-2-nitrobenzonitrile
Catalog No.:BCC8710
CAS No.:675126-26-8
- Thapsigargin
Catalog No.:BCC6952
CAS No.:67526-95-8
- Alpha-caryophyllene
Catalog No.:BCN3877
CAS No.:6753-98-6
- Helenalin
Catalog No.:BCN8073
CAS No.:6754-13-8
Oral vanoxerine prevents reinduction of atrial tachyarrhythmias: preliminary results.[Pubmed:21615815]
J Cardiovasc Electrophysiol. 2011 Nov;22(11):1266-73.
BACKGROUND: Vanoxerine is a promising, new, investigational antiarrhythmic drug. The purpose of this study was to test the hypothesis that oral dosing of Vanoxerine would first terminate induced atrial flutter (AFL) and atrial fibrillation (AF), and then prevent their reinduction. METHODS: In 5 dogs with sterile pericarditis, on the fourth day after creating the pericarditis, we performed electrophysiologic (EP) studies at baseline, measuring atrial excitability, refractoriness (AERP), and conduction time (CT) when pacing from the right atrial appendage, Bachmann's bundle (BB), and the posteroinferior left atrium at cycle lengths (CLs) of 400, 300, and 200 ms. Then, after induction of AFL or AF, all dogs received hourly oral doses of Vanoxerine: 90 mg, followed by 180 mg and 270 mg. Blood was obtained to determine plasma Vanoxerine concentrations at baseline, every 30 minutes, when neither AFL nor AF were inducible, and, finally, 1 hour after the 270 mg dose. Then we repeated the baseline EP studies. RESULTS: Four dogs had inducible, sustained AFL, and 1 dog only had induced, nonsustained AF. In 4 AFL episodes, oral Vanoxerine terminated the AFL and then rendered it noninducible after an average of 111 minutes (range 75-180 minutes) after the first dose was administered. The mean Vanoxerine plasma level at the point of noninducibility was 84 ng/mL, with a narrow range of 76-99 ng/mL. In the dog with induced, nonsustained AF, it was no longer inducible at a drug level of 75 ng/mL. Vanoxerine did not significantly (1) prolong the AERP except at BB, and then only at the faster pacing CLs; (2) change atrial excitability thresholds; (3) prolong atrial conduction time, the PR interval, the QRS complex or the QT interval. CONCLUSIONS: Orally administered Vanoxerine effectively terminated AFL and rendered it noninducible. It also suppressed inducibility of nonsustained AF. These effects occurred at consistent plasma drug levels. Vanoxerine's insignificant or minimal effects on measured electrophysiologic parameters are consistent with little proarrhythmic risk.
Randomized, double-blind, placebo-controlled study to evaluate the safety and efficacy of a single oral dose of vanoxerine for the conversion of subjects with recent onset atrial fibrillation or flutter to normal sinus rhythm: RESTORE SR.[Pubmed:27108936]
Heart Rhythm. 2016 Sep;13(9):1777-83.
BACKGROUND: Vanoxerine is an oral, 1,4-dialkylpiperazine derivative antiarrhythmic drug being evaluated for pharmacological cardioversion of atrial fibrillation (AF). OBJECTIVE: The purpose of this study was to evaluate the safety and efficacy of Vanoxerine for the restoration of sinus rhythm in subjects with recent onset AF or atrial flutter (AFL). METHODS: RESTORE SR (randomized, double-blind, placebo-controlled study to evaluate the safety and efficacy of a single oral dose of Vanoxerine for the conversion of subjects with recent onset atrial fibrillation or flutter to normal sinus rhythm) was a prospective, multinational, randomized, double-blind, placebo-controlled trial that randomized subjects to a single oral dose of Vanoxerine 400 mg or placebo (2:1 allocation). RESULTS: A total of 41 subjects were randomized in the study (placebo [n = 15] and Vanoxerine [n = 26]). The study was terminated prematurely because of safety concerns. Overall, 61% (23 of 38) of the treated cohort had a history of AF/AFL and 66% (27 of 41) had structural heart disease (SHD). The primary efficacy end point-conversion to sinus rhythm through 24 hours-occurred in 20% (3 of 15) in the placebo arm vs 69% (18 of 26) in the Vanoxerine arm (P = .0024). The mean length of stay was 4.2 +/- 2.9 days in the placebo arm vs 4.7 +/- 3.2 days in the Vanoxerine arm (P = .6561). The primary safety end point (all-cause death, ventricular fibrillation/tachycardia requiring intervention, or torsades de pointes) occurred in no patient in the placebo arm vs 11.5% (3 of 26) in the Vanoxerine arm. All 3 patients had torsades de pointes and underlying SHD. CONCLUSION: Vanoxerine is an oral, mixed ion channel blocker with IKr, INa, and L-type calcium channel activity. While oral therapy with 400 mg of Vanoxerine appears effective for the termination of recent onset AF/AFL, its use was associated with a significant risk of ventricular proarrhythmia in patients with SHD.
COR-ART: A multicenter, randomized, double-blind, placebo-controlled dose-ranging study to evaluate single oral doses of vanoxerine for conversion of recent-onset atrial fibrillation or flutter to normal sinus rhythm.[Pubmed:25684233]
Heart Rhythm. 2015 Jun;12(6):1105-12.
BACKGROUND: Restoration of sinus rhythm (SR) in patients with atrial fibrillation/atrial flutter (AF/AFL) is limited principally to direct current cardioversion. The multi-ion channel blocker Vanoxerine may prove an effective alternative. OBJECTIVE: The purpose of this study was to assess Vanoxerine, a 1,4-dialkylpiperazine derivative, for acute conversion of recent-onset, symptomatic AF and AFL. METHODS: One hundred four subjects with symptomatic AF/AFL for <7 days were randomized sequentially to single oral doses of Vanoxerine 200, 300, and 400 mg or placebo. Holter monitors were examined for conversion to SR and proarrhythmia through >/=24 hours. RESULTS: Conversion to SR was dose related: 18.2%, 44.0%, and 52.0% within 4 hours, and 59.1%, 64.0%, and 84.0% within 24 hours, for the 200-, 300-, and 400-mg groups, respectively. This was significantly higher than placebo for the 300- and 400-mg groups within 4 hours (12.5% for placebo; P = .0138 and P = .0028, respectively) and for all doses within 24 hours (31.3% for placebo; P = .0421, P = .0138, P = .0001 for 200-, 300-, and 400-mg Vanoxerine groups, respectively). Although Vanoxerine caused significant dose-dependent QTcF (QT correction by Fridericia) prolongation, monomorphic or polymorphic ventricular tachycardia did not occur. Adverse events were mild and self-limited, with only the highest dose having a greater frequency than placebo. CONCLUSION: Oral Vanoxerine converted AF/AFL to SR at a high rate, was well tolerated, and caused no ventricular proarrhythmia.
Quantitative Profiling of the Effects of Vanoxerine on Human Cardiac Ion Channels and its Application to Cardiac Risk.[Pubmed:26616666]
Sci Rep. 2015 Nov 30;5:17623.
Vanoxerine has been in clinical trials for Parkinsonism, depression and cocaine addiction but lacked efficacy. Although a potent blocker of hERG, it produced no serious adverse events. We attributed the unexpected result to offsetting Multiple Ion Channel Effects (MICE). Vanoxerine's effects were strongly frequency-dependent and we repositioned it for treatment of atrial fibrillation and flutter. Vanoxerine terminated AF/AFL in an animal model and a dose-ranging clinical trial. Reversion to normal rhythm was associated with QT prolongation yet absent proarrhythmia markers for Torsade de Pointes (TdP). To understand the QT/TdP discordance, we used quantitative profiling and compared Vanoxerine with dofetilide, a selective hERG-blocking torsadogen used for intractable AF, verapamil, a non-torsadogenic MICE comparator and bepridil, a torsadogenic MICE comparator. At clinically relevant concentrations, verapamil blocked hCav1.2 and hERG, as did Vanoxerine and bepridil both of which also blocked hNav1.5. In acute experiments and simulations, dofetilide produced early after depolarizations (EADs) and arrhythmias, whereas verapamil, Vanoxerine and bepridil produced no proarrhythmia markers. Of the MICE drugs only bepridil inhibited hERG trafficking following overnight exposure. The results are consistent with the emphasis on MICE of the CiPA assay. Additionally we propose that trafficking inhibition of hERG be added to CiPA.


