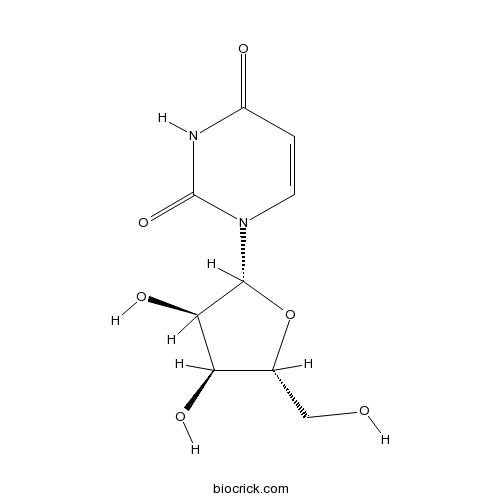
UridineCAS# 58-96-8 |
- CX-5461
Catalog No.:BCC3700
CAS No.:1138549-36-6
- Gemcitabine HCl
Catalog No.:BCC1076
CAS No.:122111-03-9
- Fludarabine Phosphate (Fludara)
Catalog No.:BCC3681
CAS No.:75607-67-9
- Bleomycin Sulfate
Catalog No.:BCC3694
CAS No.:9041-93-4
Quality Control & MSDS
Number of papers citing our products

Chemical structure

3D structure
| Cas No. | 58-96-8 | SDF | Download SDF |
| PubChem ID | 6029 | Appearance | Powder |
| Formula | C9H12N2O6 | M.Wt | 244.2 |
| Type of Compound | Alkaloids | Storage | Desiccate at -20°C |
| Synonyms | β-Uridine;Uracil riboside;Uridin | ||
| Solubility | H2O : ≥ 100 mg/mL (409.50 mM) DMSO : 100 mg/mL (409.50 mM; Need ultrasonic) *"≥" means soluble, but saturation unknown. | ||
| Chemical Name | 1-[(2R,3R,4S,5R)-3,4-dihydroxy-5-(hydroxymethyl)oxolan-2-yl]pyrimidine-2,4-dione | ||
| SMILES | C1=CN(C(=O)NC1=O)C2C(C(C(O2)CO)O)O | ||
| Standard InChIKey | DRTQHJPVMGBUCF-XVFCMESISA-N | ||
| Standard InChI | InChI=1S/C9H12N2O6/c12-3-4-6(14)7(15)8(17-4)11-2-1-5(13)10-9(11)16/h1-2,4,6-8,12,14-15H,3H2,(H,10,13,16)/t4-,6-,7-,8-/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Uridine has antidepressant-like effects, and it has protective effects against drug-induced fatty liver. Uridine can increase the rate of potassium transport in mitochondria isolated from liver of low resistant rats, and inhibitors of the channel prevent the channel activating effect of Uridine. Uridine has inhibition of p53-dependent intestinal apoptosis initiated by 5-fluorouracil. |
| Targets | ATPase | Potassium Channel | p53 |
| In vivo | The effect of uridine on the endurance of animals with different resistance to physical stress: the role of mitochondrial ATP-dependent potassium channel.[Pubmed: 25730977]Biofizika. 2014 Sep-Oct;59(5):941-5.The effect of a metabolic precursor of natural activator of mitochondrial ATP-dependent potassium channel (mitochondrial K+(ATP))--Uridine on animal's endurance to physical stress was studied.
Uridine prevents tamoxifen-induced liver lipid droplet accumulation.[Pubmed: 24887406 ]BMC Pharmacol Toxicol. 2014 May 23;15:27.Tamoxifen, an agonist of estrogen receptor, is widely prescribed for the prevention and long-term treatment of breast cancer. A side effect of tamoxifen is fatty liver, which increases the risk for non-alcoholic fatty liver disease. Prevention of tamoxifen-induced fatty liver has the potential to improve the safety of long-term tamoxifen usage.
Inhibition by uridine but not thymidine of p53-dependent intestinal apoptosis initiated by 5-fluorouracil: Evidence for the involvement of RNA perturbation.[Reference: WebLink]Proc Natl Acad Sci U S A. 1997 Mar 4; 94(5): 1795–1799.The epithelia from the crypts of the intestine are exquisitely sensitive to metabolic perturbation and undergo cell death with the classical morphology of apoptosis.
|
| Animal Research | Antidepressant-like effects of uridine and omega-3 fatty acids are potentiated by combined treatment in rats.[Pubmed: 15705349]Biol Psychiatry. 2005 Feb 15;57(4):343-50.Brain phospholipid metabolism and membrane fluidity may be involved in the pathophysiology of mood disorders. We showed previously that cytidine, which increases phospholipid synthesis, has antidepressant-like effects in the forced swim test (FST) in rats, a model used in depression research. Because cytidine and Uridine both stimulate synthesis of cytidine 5'-diphosphocholine (CDP-choline, a critical substrate for phospholipid synthesis), we examined whether Uridine would also produce antidepressant-like effects in rats. We also examined the effects of omega-3 fatty acids (OMG), which increase membrane fluidity and reportedly have antidepressant effects in humans, alone and in combination with Uridine.
|
| Structure Identification | Molecules. 2014 Apr 4;19(4):4313-25.Synthesis of extended uridine phosphonates derived from an allosteric P2Y2 receptor ligand.[Pubmed: 24714193]In this study we report the synthesis of C5/C6-fused Uridine phosphonates that are structurally related to earlier reported allosteric P2Y2 receptor ligands.
ACS Comb Sci. 2014 May 12;16(5):232-7.Parallel solution-phase synthesis and general biological activity of a uridine antibiotic analog library.[Pubmed: 24661222 ]A small library of ninety four Uridine antibiotic analogs was synthesized, under the Pilot Scale Library (PSL) Program of the NIH Roadmap initiative, from amine 2 and carboxylic acids 33 and 77 in solution-phase fashion.
|

Uridine Dilution Calculator

Uridine Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 4.095 mL | 20.475 mL | 40.95 mL | 81.9001 mL | 102.3751 mL |
| 5 mM | 0.819 mL | 4.095 mL | 8.19 mL | 16.38 mL | 20.475 mL |
| 10 mM | 0.4095 mL | 2.0475 mL | 4.095 mL | 8.19 mL | 10.2375 mL |
| 50 mM | 0.0819 mL | 0.4095 mL | 0.819 mL | 1.638 mL | 2.0475 mL |
| 100 mM | 0.041 mL | 0.2048 mL | 0.4095 mL | 0.819 mL | 1.0238 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Uridine, Trisodium Salt is an energy-rich precursor in the enzymatic biosynthesis of RNA, a potent vasodilator, and induces contractile responses in some tissues.
- alpha-Tocopherol acetate
Catalog No.:BCN5803
CAS No.:58-95-7
- Chlorothiazide
Catalog No.:BCC3752
CAS No.:58-94-6
- Hydrochlorothiazide
Catalog No.:BCC4786
CAS No.:58-93-5
- D-(+)-Xylose
Catalog No.:BCN1010
CAS No.:58-86-6
- Biotin
Catalog No.:BCC3585
CAS No.:58-85-5
- Papaverine
Catalog No.:BCC8230
CAS No.:58-74-2
- Inosine
Catalog No.:BCN3841
CAS No.:58-63-9
- Adenosine
Catalog No.:BCN5796
CAS No.:58-61-7
- Puromycin aminonucleoside
Catalog No.:BCC1873
CAS No.:58-60-6
- Puromycin dihydrochloride
Catalog No.:BCC7860
CAS No.:58-58-2
- Pyridoxine HCl
Catalog No.:BCC4835
CAS No.:58-56-0
- Theophylline
Catalog No.:BCN1258
CAS No.:58-55-9
- 6-Aminoquinoline
Catalog No.:BCC8766
CAS No.:580-15-4
- 3-Aminoquinoline
Catalog No.:BCC8620
CAS No.:580-17-6
- 2-Aminoquinoline
Catalog No.:BCC8555
CAS No.:580-22-3
- Matairesinol
Catalog No.:BCN5789
CAS No.:580-72-3
- Epicorynoxidine
Catalog No.:BCN7554
CAS No.:58000-48-9
- HOKU-81
Catalog No.:BCC1634
CAS No.:58020-43-2
- Averantin
Catalog No.:BCN7027
CAS No.:5803-62-3
- 24, 25-Dihydroxy VD2
Catalog No.:BCC1302
CAS No.:58050-55-8
- Miltefosine
Catalog No.:BCC4360
CAS No.:58066-85-6
- trans-3,4-Methylenedioxycinnamyl alcohol
Catalog No.:BCN1410
CAS No.:58095-76-4
- α-MSH
Catalog No.:BCC7420
CAS No.:581-05-5
- Suberosin
Catalog No.:BCN5791
CAS No.:581-31-7
Antidepressant-like effects of uridine and omega-3 fatty acids are potentiated by combined treatment in rats.[Pubmed:15705349]
Biol Psychiatry. 2005 Feb 15;57(4):343-50.
BACKGROUND: Brain phospholipid metabolism and membrane fluidity may be involved in the pathophysiology of mood disorders. We showed previously that cytidine, which increases phospholipid synthesis, has antidepressant-like effects in the forced swim test (FST) in rats, a model used in depression research. Because cytidine and Uridine both stimulate synthesis of cytidine 5'-diphosphocholine (CDP-choline, a critical substrate for phospholipid synthesis), we examined whether Uridine would also produce antidepressant-like effects in rats. We also examined the effects of omega-3 fatty acids (OMG), which increase membrane fluidity and reportedly have antidepressant effects in humans, alone and in combination with Uridine. METHODS: We first examined the effects of Uridine injections alone and dietary supplementation with OMG alone in the FST. We then combined sub-effective treatment regimens of Uridine and OMG to determine whether these agents would be more effective if administered together. RESULTS: Uridine dose-dependently reduced immobility in the FST, an antidepressant-like effect. Dietary supplementation with OMG reduced immobility when given for 30 days, but not for 3 or 10 days. A sub-effective dose of Uridine reduced immobility in rats given sub-effective dietary supplementation with OMG. CONCLUSIONS: Uridine and OMG each have antidepressant-like effects in rats. Less of each agent is required for effectiveness when the treatments are administered together.
Parallel solution-phase synthesis and general biological activity of a uridine antibiotic analog library.[Pubmed:24661222]
ACS Comb Sci. 2014 May 12;16(5):232-7.
A small library of ninety four Uridine antibiotic analogs was synthesized, under the Pilot Scale Library (PSL) Program of the NIH Roadmap initiative, from amine 2 and carboxylic acids 33 and 77 in solution-phase fashion. Diverse aldehyde, sulfonyl chloride, and carboxylic acid reactant sets were condensed to 2, leading after acid-mediated hydrolysis, to the targeted compounds 3-32 in good yields and high purity. Similarly, treatment of 33 with diverse amines and sulfonamides gave 34-75. The coupling of the amino terminus of d-phenylalanine methyl ester to the free 5'-carboxylic acid moiety of 33 followed by sodium hydroxide treatment led to carboxylic acid analog 77. Hydrolysis of this material gave analog 78. The intermediate 77 served as the precursor for the preparation of novel dipeptidyl Uridine analogs 79-99 through peptide coupling reactions to diverse amine reactants. None of the described compounds show significant anticancer or antimalarial acivity. A number of samples exhibited a variety of promising inhibitory, agonist, antagonist, or activator properties with enzymes and receptors in primary screens supplied and reported through the NIH MLPCN program.
Synthesis of extended uridine phosphonates derived from an allosteric P2Y2 receptor ligand.[Pubmed:24714193]
Molecules. 2014 Apr 4;19(4):4313-25.
In this study we report the synthesis of C5/C6-fused Uridine phosphonates that are structurally related to earlier reported allosteric P2Y2 receptor ligands. A silyl-Hilbert-Johnson reaction of six quinazoline-2,4-(1H,3H)-dione-like base moieties with a suitable ribofuranosephosphonate afforded the desired analogues after full deprotection. In contrast to the parent 5-(4-fluoropheny)Uridine phosphonate, the present extended-base Uridine phosphonates essentially failed to modulate the P2Y2 receptor.
Uridine prevents tamoxifen-induced liver lipid droplet accumulation.[Pubmed:24887406]
BMC Pharmacol Toxicol. 2014 May 23;15:27.
BACKGROUND: Tamoxifen, an agonist of estrogen receptor, is widely prescribed for the prevention and long-term treatment of breast cancer. A side effect of tamoxifen is fatty liver, which increases the risk for non-alcoholic fatty liver disease. Prevention of tamoxifen-induced fatty liver has the potential to improve the safety of long-term tamoxifen usage. METHODS: Uridine, a pyrimidine nucleoside with reported protective effects against drug-induced fatty liver, was co-administered with tamoxifen in C57BL/6J mice. Liver lipid levels were evaluated with lipid visualization using coherent anti-Stokes Raman scatting (CARS) microscopy, biochemical assay measurement of triacylglyceride (TAG), and liquid chromatography coupled with mass spectrometry (LC-MS) measurement of membrane phospholipid. Blood TAG and cholesterol levels were measured. Mitochondrial respiration of primary hepatocytes in the presence of tamoxifen and/or Uridine was evaluated by measuring oxygen consumption rate with an extracellular flux analyzer. Liver protein lysine acetylation profiles were evaluated with 1D and 2D Western blots. In addition, the relationship between endogenous Uridine levels, fatty liver, and tamoxifen administration was evaluated in transgenic mice UPase1-/-and UPase1-TG. RESULTS: Uridine co-administration prevented tamoxifen-induced liver lipid droplet accumulation in mice. The most prominent effect of Uridine co-administration with tamoxifen was the stimulation of liver membrane phospholipid biosynthesis. Uridine had no protective effect against tamoxifen-induced impairment to mitochondrial respiration of primary hepatocytes or liver TAG and cholesterol export. Uridine had no effect on tamoxifen-induced changes to liver protein acetylation profile. Transgenic mice UPase1-/-with increased pyrimidine salvage activity were protected against tamoxifen-induced liver lipid droplet accumulation. In contrast, UPase1-TG mice with increased pyrimidine catabolism activity had intrinsic liver lipid droplet accumulation, which was aggravated following tamoxifen administration. CONCLUSION: Uridine co-administration was effective at preventing tamoxifen-induced liver lipid droplet accumulation. The ability of Uridine to prevent tamoxifen-induced fatty liver appeared to depend on the pyrimidine salvage pathway, which promotes biosynthesis of membrane phospholipid.
[The effect of uridine on the endurance of animals with different resistance to physical stress: the role of mitochondrial ATP-dependent potassium channel].[Pubmed:25730977]
Biofizika. 2014 Sep-Oct;59(5):941-5.
The effect of a metabolic precursor of natural activator of mitochondrial ATP-dependent potassium channel (mitochondrial K+(ATP))--Uridine on animal's endurance to physical stress was studied. The endurance was determined by recording the time period during which the rat loaded with a plummet of 20% of body weight can swim until physical exhaustion at 32 degrees C. It was found that highly resistant animals swam until exhaustion for 7.40 +/- 0.35 min, whereas low resistant rats hold out 2.07 +/- 0.10 min only. The injection of Uridine influenced the swimming time of the animals, increasing it twofold in low-resistant rats. The effect of Uridine was decreased by injection of inhibitors of mitochondrial K+(ATP) channel. It was found that the injection of Uridine into low resistant rats increased the rate of potassium transport in mitochondria isolated from liver of these rats, and inhibitors of the channel prevent the channel activating effect of Uridine. The role of mitochondrial K+(ATP) cannel in the formation of animal's resistance to physical stress and protection of tissues from hypoxia is discussed.


