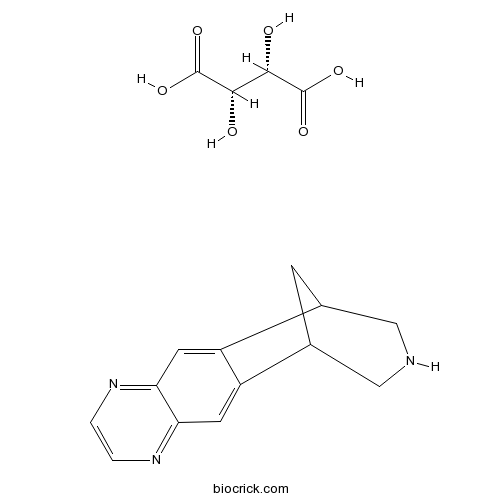
Varenicline tartrateSubtype-selective agonist of α4β2 nicotinic receptors,orally active CAS# 375815-87-5 |
- A-867744
Catalog No.:BCC1324
CAS No.:1000279-69-5
- Rocuronium Bromide
Catalog No.:BCC1068
CAS No.:119302-91-9
- Rivastigmine
Catalog No.:BCC1900
CAS No.:123441-03-2
Quality Control & MSDS
Number of papers citing our products

Chemical structure

3D structure
| Cas No. | 375815-87-5 | SDF | Download SDF |
| PubChem ID | 170362 | Appearance | Powder |
| Formula | C17H19N3O6 | M.Wt | 361.35 |
| Type of Compound | Alkaloids | Storage | Desiccate at -20°C |
| Synonyms | CP 526555-18; Champix tartrate; Chantix tartrate | ||
| Solubility | Soluble to 100 mM in water and to 50 mM in DMSO | ||
| SMILES | C1C2CNCC1C3=CC4=NC=CN=C4C=C23.C(C(C(=O)O)O)(C(=O)O)O | ||
| Standard InChIKey | TWYFGYXQSYOKLK-WUUYCOTASA-N | ||
| Standard InChI | InChI=1S/C13H13N3.C4H6O6/c1-2-16-13-5-11-9-3-8(6-14-7-9)10(11)4-12(13)15-1;5-1(3(7)8)2(6)4(9)10/h1-2,4-5,8-9,14H,3,6-7H2;1-2,5-6H,(H,7,8)(H,9,10)/t;1-,2-/m.0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Varenicline tartrate appears to be a safe and well-tolerated opportunistic treatment for inpatient smokers who have related chronic disease, suggests it be considered as part of standard care in the hospital setting.Varenicline significantly reduces alcohol consumption and craving, making it a potentially viable option for the treatment of alcohol dependence. |
| In vivo | Safety of varenicline tartrate and counseling versus counseling alone for smoking cessation: a randomized controlled trial for inpatients (STOP study).[Pubmed: 25031315]Nicotine Tob Res. 2014 Nov;16(11):1495-502. Inpatient medical settings offer an opportunistic environment for initiating smoking cessation interventions to patients reflecting on their health. Current evidence has shown the superior efficacy of Varenicline tartrate (VT) for smoking cessation compared with other tobacco cessation therapies; however, recent evidence also has highlighted concerns about the safety and tolerability of Varenicline tartrate. Given these apprehensions, we aimed to evaluate the safety and effectiveness of Varenicline tartrate plus quitline-counseling compared to quitline-counseling alone in the inpatient medical setting. A double-blind, placebo-controlled trial assessing the efficacy of varenicline tartrate for alcohol dependence.[Pubmed: 23728065]J Addict Med. 2013 Jul-Aug;7(4):277-86.To assess the efficacy and safety of Varenicline tartrate (Chantix) for the treatment of alcohol dependence. Varenicline tartrate is a partial α4β2 nicotinic acetylcholine agonist approved by the Food and Drug Administration for smoking cessation. It has reduced drinking in animal studies and in small studies of humans who were both heavy drinkers and smokers. This is the first multisite clinical trial of varenicline in a population of smokers and nonsmokers with alcohol dependence. Safety and tolerability of varenicline tartrate (Champix(®)/Chantix(®)) for smoking cessation in HIV-infected subjects: a pilot open-label study.[Pubmed: 22007690]AIDS Patient Care STDS. 2012 Jan;26(1):12-9.The prevalence of smoking in HIV-infected subjects is high. As a smoking cessation aid, Varenicline tartrate (Champix(®), Pfizer, Saint-Laurent, QC, Canada or Chantix(®), Pfizer, Mission, KS) has not been previously evaluated in HIV-infected smokers. |

Varenicline tartrate Dilution Calculator

Varenicline tartrate Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.7674 mL | 13.837 mL | 27.674 mL | 55.348 mL | 69.185 mL |
| 5 mM | 0.5535 mL | 2.7674 mL | 5.5348 mL | 11.0696 mL | 13.837 mL |
| 10 mM | 0.2767 mL | 1.3837 mL | 2.7674 mL | 5.5348 mL | 6.9185 mL |
| 50 mM | 0.0553 mL | 0.2767 mL | 0.5535 mL | 1.107 mL | 1.3837 mL |
| 100 mM | 0.0277 mL | 0.1384 mL | 0.2767 mL | 0.5535 mL | 0.6919 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Orally active, subtype-selective partial agonist at α4β2 nicotinic receptors (Ki values are 0.06, 240, 322 and 3540 nM for α4β2, α3β4, α7, α1βγδ receptors respectively). Reduces nicotine-evoked dopamine release in vitro and decreases nicotine self-adminis
- Phorbol 12,13-dibutyrate
Catalog No.:BCC7870
CAS No.:37558-16-0
- Boc-N-Me-Phe-OH
Catalog No.:BCC2615
CAS No.:37553-65-4
- Boc-Ala(4-pyridyl)-OH
Catalog No.:BCC3326
CAS No.:37535-57-2
- 7'(Z)-(8''R,8'''R)-epi-salvianolic acid E
Catalog No.:BCC3319
CAS No.:
- 3-(2-Pyridyl)-D-Alanine
Catalog No.:BCC2655
CAS No.:37535-52-7
- H- Ala(2-pyridyl)-OH.2HCl
Catalog No.:BCC3318
CAS No.:37535-51-6 net
- 3-(2-Pyridyl)-Alanine
Catalog No.:BCC2656
CAS No.:37535-51-6
- H-D-Ala(4-pyridyl)-OH.HCl
Catalog No.:BCC3325
CAS No.:37535-49-2
- LY451395
Catalog No.:BCC5377
CAS No.:375345-95-2
- 2-Chlorocinnamic acid
Catalog No.:BCN5036
CAS No.:3752-25-8
- Amikacin
Catalog No.:BCC5206
CAS No.:37517-28-5
- DHP Linker
Catalog No.:BCC2830
CAS No.:3749-36-8
- Furaltadone HCl
Catalog No.:BCC4662
CAS No.:3759-92-0
- Maraviroc
Catalog No.:BCC3675
CAS No.:376348-65-1
- 5,7,2',4'-Tetrahydroxy-3-geranylflavone
Catalog No.:BCN1451
CAS No.:376361-87-4
- 7,4'-Dihydroxy-3'-prenylflavan
Catalog No.:BCN5428
CAS No.:376361-96-5
- 4'-O-Demethylbroussonin A
Catalog No.:BCN7364
CAS No.:376361-97-6
- 1-(4-Hydroxy-2-methoxyphenyl)-3-(4-hydroxy-3-prenylphenyl)propane
Catalog No.:BCN1450
CAS No.:376362-03-7
- BRD 7389
Catalog No.:BCC8090
CAS No.:376382-11-5
- LY450108
Catalog No.:BCC1725
CAS No.:376594-67-1
- 8-Acetonyldihydrosanguinarine
Catalog No.:BCN5429
CAS No.:37687-34-6
- SU 9516
Catalog No.:BCC2398
CAS No.:377090-84-1
- Dehydroabietinol
Catalog No.:BCN5430
CAS No.:3772-55-2
- Totaradiol
Catalog No.:BCN5431
CAS No.:3772-56-3
Safety of varenicline tartrate and counseling versus counseling alone for smoking cessation: a randomized controlled trial for inpatients (STOP study).[Pubmed:25031315]
Nicotine Tob Res. 2014 Nov;16(11):1495-502.
INTRODUCTION: Inpatient medical settings offer an opportunistic environment for initiating smoking cessation interventions to patients reflecting on their health. Current evidence has shown the superior efficacy of Varenicline tartrate (VT) for smoking cessation compared with other tobacco cessation therapies; however, recent evidence also has highlighted concerns about the safety and tolerability of VT. Given these apprehensions, we aimed to evaluate the safety and effectiveness of VT plus quitline-counseling compared to quitline-counseling alone in the inpatient medical setting. METHODS: Adult patients (n = 392, 20-75 years) admitted with a smoking-related illnesses to 3 hospitals were randomized to receive either 12 weeks of Varenicline tartrate (titrated from 0.5mg daily to 1mg twice daily) plus quitline-counseling (VT+C), (n = 196) or quitline-counseling alone (n = 196). RESULTS: VT was well tolerated in the inpatient setting among subjects admitted with acute smoking-related illnesses (mean age 52.8+/-2.89 and 53.7+/-2.77 years in the VT+C and counseling alone groups, respectively). The most common self-reported adverse event during the 12-week treatment phase was nausea (16.3% in the VT+C group compared with 1.5% in the counseling alone group). Thirteen deaths occurred during the study period (n = 6 were in the VT+C arm compared with n = 7 in the counseling alone arm). All of these subjects had known comorbidities or developed underlying comorbidities. CONCLUSIONS: VT appears to be a safe and well-tolerated opportunistic treatment for inpatient smokers who have related chronic disease. Based on the proven efficacy of varenicline from outpatient studies and our recent inpatient evidence, we suggest it be considered as part of standard care in the hospital setting.
Safety and tolerability of varenicline tartrate (Champix((R))/Chantix((R))) for smoking cessation in HIV-infected subjects: a pilot open-label study.[Pubmed:22007690]
AIDS Patient Care STDS. 2012 Jan;26(1):12-9.
The prevalence of smoking in HIV-infected subjects is high. As a smoking cessation aid, varenicline (Champix((R)), Pfizer, Saint-Laurent, QC, Canada or Chantix((R)), Pfizer, Mission, KS) has not been previously evaluated in HIV-infected smokers. In this multicenter pilot open label study, varenicline 1.0 mg was used twice daily for 12 weeks with dose titration in the first week. Adverse events (AEs) during the treatment period were recorded. Changes from baseline in laboratory tests, vital signs, daily cigarette consumption, nicotine dependence, and withdrawal were measured through week 24. Self-reported abstinence was validated by serum cotinine at week 12. We enrolled 36 subjects with a mean of 29 pack-years of smoking and a minimum of 4 cigarettes per day. All but 1 were male, 33 (92%) were white. The most frequently reported AEs were nausea (33%), abnormal dreams (31%), affect lability (19%), and insomnia (19%). Six (17%) subjects discontinued varenicline due to AEs. No grade 3/4 laboratory abnormalities or serious AEs occurred during the study. There was no significant change in HIV viral load. CD4 counts increased by 69 cells/mm3 (p = 0.001) at week 24. Serum cotinine-verified 4-week continuous abstinence rate through weeks 9-12 was 42% (95% confidence interval [CI]: 26-58%). AEs and abstinence rates were comparable to those in published randomized controlled trials conducted in generally healthy HIV-negative smokers. Varenicline was safe and appears effective among HIV-infected smokers in this exploratory study, although AEs were common. The most common AE was nausea, with no adverse effect on HIV treatment outcome. Close monitoring of liver enzymes and blood pressure is recommended for HIV-positive smokers taking varenicline.
A double-blind, placebo-controlled trial assessing the efficacy of varenicline tartrate for alcohol dependence.[Pubmed:23728065]
J Addict Med. 2013 Jul-Aug;7(4):277-86.
OBJECTIVES: To assess the efficacy and safety of varenicline (Chantix) for the treatment of alcohol dependence. Varenicline is a partial alpha4beta2 nicotinic acetylcholine agonist approved by the Food and Drug Administration for smoking cessation. It has reduced drinking in animal studies and in small studies of humans who were both heavy drinkers and smokers. This is the first multisite clinical trial of varenicline in a population of smokers and nonsmokers with alcohol dependence. METHODS: Men and women (n = 200) meeting the criteria for alcohol dependence were recruited across 5 clinical sites. Patients received double-blind varenicline or placebo and a computerized behavioral intervention. Varenicline was titrated during the first week to 2 mg/d, which was maintained during weeks 2 to 13. RESULTS: The varenicline group had significantly lower weekly percent heavy drinking days (primary outcome) (adjusted mean difference = 10.4), drinks per day, drinks per drinking day, and alcohol craving compared with the placebo group (P < 0.05). The average treatment effect on alcohol use was similar for smokers and nonsmokers. Varenicline was well-tolerated; adverse events were expected and mild. CONCLUSIONS: Varenicline significantly reduced alcohol consumption and craving, making it a potentially viable option for the treatment of alcohol dependence.
Preclinical pharmacology of the alpha4beta2 nAChR partial agonist varenicline related to effects on reward, mood and cognition.[Pubmed:19501054]
Biochem Pharmacol. 2009 Oct 1;78(7):813-24.
The pharmacological properties and pharmacokinetic profile of the alpha4beta2 nicotinic acetylcholine receptor (nAChR) partial agonist varenicline provide an advantageous combination of free brain levels and functional potencies at the target receptor that for a large part explain its efficacy as a smoking cessation aid. Since alpha4beta2 and other nAChR subtypes play important roles in mediating central processes that control reward, mood, cognition and attention, there is interest in examining the effects of selective nAChR ligands such as varenicline in preclinical animal models that assess these behaviors. Here we describe results from studies on varenicline's effects in animal models of addiction, depression, cognition and attention and discuss these in the context of recently published preclinical and preliminary clinical studies that collected data on varenicline's effects on mood, cognition and alcohol abuse disorder. Taken together, the preclinical and the limited clinical data show beneficial effects of varenicline, but further clinical studies are needed to evaluate whether the preclinical effects observed in animal models are translatable to the clinic.
Pharmacological profile of the alpha4beta2 nicotinic acetylcholine receptor partial agonist varenicline, an effective smoking cessation aid.[Pubmed:17157884]
Neuropharmacology. 2007 Mar;52(3):985-94.
The preclinical pharmacology of the alpha4beta2 nicotinic acetylcholine receptor (nAChR) partial agonist varenicline, a novel smoking cessation agent is described. Varenicline binds with subnanomolar affinity only to alpha4beta2 nAChRs and in vitro functional patch clamp studies in HEK cells expressing nAChRs show that varenicline is a partial agonist with 45% of nicotine's maximal efficacy at alpha4beta2 nAChRs. In neurochemical models varenicline has significantly lower (40-60%) efficacy than nicotine in stimulating [(3)H]-dopamine release from rat brain slices in vitro and in increasing dopamine release from rat nucleus accumbens in vivo, while it is more potent than nicotine. In addition, when combined with nicotine, varenicline effectively attenuates the nicotine-induced dopamine release to the level of the effect of varenicline alone, consistent with partial agonism. Finally, varenicline reduces nicotine self-administration in rats and supports lower self-administration break points than nicotine. These data suggest that varenicline can reproduce to some extent the subjective effects of smoking by partially activating alpha4beta2 nAChRs, while preventing full activation of these receptors by nicotine. Based on these findings, varenicline was advanced into clinical development and recently shown to be an effective and safe aid for smoking cessation treatment.
Varenicline: an alpha4beta2 nicotinic receptor partial agonist for smoking cessation.[Pubmed:15887955]
J Med Chem. 2005 May 19;48(10):3474-7.
Herein we describe a novel series of compounds from which varenicline (1, 6,7,8,9-tetrahydro-6,10-methano-6H-pyrazino[2,3-h][3]benzazepine) has been identified for smoking cessation. Neuronal nicotinic acetylcholine receptors (nAChRs) mediate the dependence-producing effects of nicotine. We have pursued alpha4beta2 nicotinic receptor partial agonists to inhibit dopaminergic activation produced by smoking while simultaneously providing relief from the craving and withdrawal syndrome that accompanies cessation attempts. Varenicline displays high alpha4beta2 nAChR affinity and the desired in vivo dopaminergic profile.


