VoreloxinTopo II inhibitor CAS# 175414-77-4 |
- Gatifloxacin
Catalog No.:BCC1064
CAS No.:112811-59-3
- Dexrazoxane HCl (ICRF-187, ADR-529)
Catalog No.:BCC1087
CAS No.:149003-01-0
- Doxorubicin (Adriamycin) HCl
Catalog No.:BCC1117
CAS No.:25316-40-9
- Etoposide
Catalog No.:BCC1151
CAS No.:33419-42-0
- Genistein
Catalog No.:BCN5499
CAS No.:446-72-0
Quality Control & MSDS
Number of papers citing our products

Chemical structure

3D structure
| Cas No. | 175414-77-4 | SDF | Download SDF |
| PubChem ID | 9952884 | Appearance | Powder |
| Formula | C18H19N5O4S | M.Wt | 401.44 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | SNS-595; Vosaroxin; AG 7352 | ||
| Solubility | Soluble in DMSO > 10 mM | ||
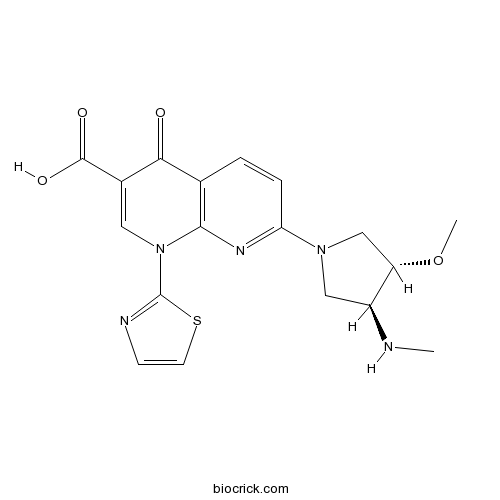
| Chemical Name | 7-[(3S,4S)-3-methoxy-4-(methylamino)pyrrolidin-1-yl]-4-oxo-1-(1,3-thiazol-2-yl)-1,8-naphthyridine-3-carboxylic acid | ||
| SMILES | CNC1CN(CC1OC)C2=NC3=C(C=C2)C(=O)C(=CN3C4=NC=CS4)C(=O)O | ||
| Standard InChIKey | XZAFZXJXZHRNAQ-STQMWFEESA-N | ||
| Standard InChI | InChI=1S/C18H19N5O4S/c1-19-12-8-22(9-13(12)27-2)14-4-3-10-15(24)11(17(25)26)7-23(16(10)21-14)18-20-5-6-28-18/h3-7,12-13,19H,8-9H2,1-2H3,(H,25,26)/t12-,13-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Voreloxin is an inhibitor of topoisomerase II. | |||||
| Targets | topoisomerase II | |||||

Voreloxin Dilution Calculator

Voreloxin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.491 mL | 12.4552 mL | 24.9103 mL | 49.8206 mL | 62.2758 mL |
| 5 mM | 0.4982 mL | 2.491 mL | 4.9821 mL | 9.9641 mL | 12.4552 mL |
| 10 mM | 0.2491 mL | 1.2455 mL | 2.491 mL | 4.9821 mL | 6.2276 mL |
| 50 mM | 0.0498 mL | 0.2491 mL | 0.4982 mL | 0.9964 mL | 1.2455 mL |
| 100 mM | 0.0249 mL | 0.1246 mL | 0.2491 mL | 0.4982 mL | 0.6228 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Voreloxin, formerly known as SNS-595 or AG-7352, is a novel naphthyridine analog, which is structurally related to the quinolone antibiotics, a chemical class not previously used for the treatment
of cancer.
In vitro: In vitro studies demonstrated voreloxin has broad anti-proliferative activity in 11 tumor cell lines, with IC50 values ranging from 0.04 to 0.97 μM. Similar activity was observed in vitro in drug-resistant cell lines, including those that overexpress P-glycoprotein [2].
In vivo: After a single intravenous dose, voreloxin concentrations in tumor were correlated with induction of the apoptosis marker caspase-3. Administration of voreloxin at 20 mg/kg weekly inhibited tumor growth (86%). Voreloxin demonstrated strong dose-dependent tumor growth inhibition (63–88%) in 10 of 11 solid tumor xenograft models [2].
Clinical trial: Voreloxin showed an acceptable safety profile with clinical activity in patients with relapsed/refractory solid tumors. The maxmum tolerence dose was schedule-dependent. Voreloxin is now in clinical studies of ovarian cancer and acute myeloid leukemia [3].
References:
[1] Tsuzuki Y, Tomita K, Shibamori K, Sato Y, Kashimoto S, Chiba K. Synthesis and structure-activity relationships of novel 7-substituted 1,4-dihydro-4-oxo-1-(2-thiazolyl)-1,8-naphthyridine-3-carboxylic acids as antitumor agents. Part 2. J Med Chem. 2004;47(8):2097-109.
[2] Hoch U, Lynch J, Sato Y, Kashimoto S, Kajikawa F, Furutani Y, Silverman JA. Voreloxin, formerly SNS-595, has potent activity against a broad panel of cancer cell lines and in vivo tumor models. Cancer Chemother Pharmacol. 2009;64(1):53-65.
[3] Advani RH, Hurwitz HI, Gordon MS, Ebbinghaus SW, Mendelson DS, Wakelee HA, Hoch U, Silverman JA, Havrilla NA, Berman CJ, Fox JA, Allen RS, Adelman DC. Voreloxin, a first-in-class anticancer quinolone derivative, in relapsed/refractory solid tumors: a report on two dosing schedules. Clin Cancer Res. 2010;16(7):2167-75.
- S 18986
Catalog No.:BCC6081
CAS No.:175340-20-2
- Fmoc-Thr(HPO3Bzl)-OH
Catalog No.:BCC3551
CAS No.:175291-56-2
- Xanthorin
Catalog No.:BCN1122
CAS No.:17526-15-7
- O4I1
Catalog No.:BCC6542
CAS No.:175135-47-4
- NQDI 1
Catalog No.:BCC2404
CAS No.:175026-96-7
- Tonabersat
Catalog No.:BCC2009
CAS No.:175013-84-0
- Parishin C
Catalog No.:BCN3813
CAS No.:174972-80-6
- Parishin B
Catalog No.:BCN3812
CAS No.:174972-79-3
- Rabdoketone B
Catalog No.:BCN6598
CAS No.:174819-51-3
- 3-Amino-4-methoxybenzamide
Catalog No.:BCC8612
CAS No.:17481-27-5
- Fmoc-Hyp(Bzl)-OH
Catalog No.:BCC3255
CAS No.:174800-02-3
- Carabrone
Catalog No.:BCN1121
CAS No.:1748-81-8
- Z-Phe(4-F)-OH
Catalog No.:BCC3221
CAS No.:17543-58-7
- Eriosematin A
Catalog No.:BCN3465
CAS No.:175448-02-9
- Fmoc-3-Pal-OH
Catalog No.:BCC2653
CAS No.:175453-07-3
- Fmoc-Phe(4-Cl)-OH
Catalog No.:BCC3173
CAS No.:175453-08-4
- Voreloxin Hydrochloride
Catalog No.:BCC2045
CAS No.:175519-16-1
- Longifloroside A
Catalog No.:BCN1123
CAS No.:175556-08-8
- Tapentadol Hydrochloride
Catalog No.:BCC9159
CAS No.:175591-09-0
- α-Conotoxin MII
Catalog No.:BCC5743
CAS No.:175735-93-0
- Lanatoside B
Catalog No.:BCN6544
CAS No.:17575-21-2
- Lanatoside C
Catalog No.:BCN6457
CAS No.:17575-22-3
- PPAHV
Catalog No.:BCC7077
CAS No.:175796-50-6
- H-Dab.HBr
Catalog No.:BCC3184
CAS No.:1758-80-1
Voreloxin, a first-in-class anticancer quinolone derivative, in relapsed/refractory solid tumors: a report on two dosing schedules.[Pubmed:20233886]
Clin Cancer Res. 2010 Apr 1;16(7):2167-75.
PURPOSE: Voreloxin, a novel replication-dependent DNA-damaging agent, intercalates DNA and inhibits topoisomerase II. Voreloxin induces site-selective DNA double-strand breaks and apoptosis. We report the phase 1 experience of Voreloxin in patients with relapsed/refractory solid tumors, including dose-limiting toxicity (DLT), maximum-tolerated dose (MTD), pharmacokinetics, and clinical activity. EXPERIMENTAL DESIGN: Two dose-escalation studies evaluated Voreloxin administered i.v. every 3 weeks (SPO-0001) or weekly for 3 weeks every 28 days (SPO-0002). In SPO-0001, patients were classified as heavily pretreated (HP) or minimally pretreated (MP) based on therapeutic history. RESULTS: In the SPO-0001 study, 41 patients (24 HP/17 MP) were treated in eight dose cohorts (3-75 mg/m(2)). At 60 mg/m(2), four HP patients experienced DLTs: grade 4 neutropenia (n = 3, one with fever) and grade 3 febrile neutropenia/pneumonia (n = 1). At 75 mg/m(2), two MP patients experienced DLTs: grade 4 neutropenia/thrombocytopenia (n = 1) or grade 2 oral thrush for >29 days (n = 1). Therefore, the MTD was 48 mg/m(2) (HP patients) and 60 mg/m(2) (MP patients). In the SPO-0002 study, 21 patients were treated in six dose cohorts (3-24 mg/m(2)). At 18 mg/m(2), two patients experienced DLTs: grade 3 neutropenia, one with pleural effusion (>14 days each). The MTD was 15 mg/m(2). Voreloxin exhibited low clearance (2 L/h/m(2)), a long terminal half-life (22 hours), and dose-proportional exposure. Overall, 31 of 62 patients had stable disease and 1 patient (ovarian cancer) had a partial response per Rustin criteria. CONCLUSIONS: Voreloxin showed an acceptable safety profile with clinical activity in patients with relapsed/refractory solid tumors. The MTD was schedule-dependent. Voreloxin is currently in clinical studies of ovarian cancer and acute myeloid leukemia.
Voreloxin is an anticancer quinolone derivative that intercalates DNA and poisons topoisomerase II.[Pubmed:20419121]
PLoS One. 2010 Apr 15;5(4):e10186.
BACKGROUND: Topoisomerase II is critical for DNA replication, transcription and chromosome segregation and is a well validated target of anti-neoplastic drugs including the anthracyclines and epipodophyllotoxins. However, these drugs are limited by common tumor resistance mechanisms and side-effect profiles. Novel topoisomerase II-targeting agents may benefit patients who prove resistant to currently available topoisomerase II-targeting drugs or encounter unacceptable toxicities. Voreloxin is an anticancer quinolone derivative, a chemical scaffold not used previously for cancer treatment. Voreloxin is completing Phase 2 clinical trials in acute myeloid leukemia and platinum-resistant ovarian cancer. This study defined Voreloxin's anticancer mechanism of action as a critical component of rational clinical development informed by translational research. METHODS/PRINCIPAL FINDINGS: Biochemical and cell-based studies established that Voreloxin intercalates DNA and poisons topoisomerase II, causing DNA double-strand breaks, G2 arrest, and apoptosis. Voreloxin is differentiated both structurally and mechanistically from other topoisomerase II poisons currently in use as chemotherapeutics. In cell-based studies, Voreloxin poisoned topoisomerase II and caused dose-dependent, site-selective DNA fragmentation analogous to that of quinolone antibacterials in prokaryotes; in contrast etoposide, the nonintercalating epipodophyllotoxin topoisomerase II poison, caused extensive DNA fragmentation. Etoposide's activity was highly dependent on topoisomerase II while Voreloxin and the intercalating anthracycline topoisomerase II poison, doxorubicin, had comparable dependence on this enzyme for inducing G2 arrest. Mechanistic interrogation with Voreloxin analogs revealed that intercalation is required for Voreloxin's activity; a nonintercalating analog did not inhibit proliferation or induce G2 arrest, while an analog with enhanced intercalation was 9.5-fold more potent. CONCLUSIONS/SIGNIFICANCE: As a first-in-class anticancer quinolone derivative, Voreloxin is a toposiomerase II-targeting agent with a unique mechanistic signature. A detailed understanding of Voreloxin's molecular mechanism, in combination with its evolving clinical profile, may advance our understanding of structure-activity relationships to develop safer and more effective topoisomerase II-targeted therapies for the treatment of cancer.
The topoisomerase II inhibitor voreloxin causes cell cycle arrest and apoptosis in myeloid leukemia cells and acts in synergy with cytarabine.[Pubmed:21134979]
Haematologica. 2011 Mar;96(3):393-9.
BACKGROUND: Topoisomerase II is essential for the maintenance of DNA integrity and the survival of proliferating cells. Topoisomerase II poisons, including etoposide and doxorubicin, inhibit enzyme-mediated DNA ligation causing the accumulation of double-stranded breaks and have been front-line drugs for the treatment of leukemia for many years. Voreloxin is a first-in-class anti-cancer quinolone derivative that intercalates DNA and inhibits topoisomerase II. The efficacy and mechanisms of action of Voreloxin in acute myeloid leukaemia were addressed in this study. DESIGN AND METHODS: Primary acute myeloid leukemia blasts (n = 88) and myeloid cell lines were used in vitro to study Voreloxin through viability assays to assess cell killing and synergy with other drugs. Apoptosis and cell cycling were assessed by flow cytometry. DNA relaxation assays were utilized to determine that Voreloxin was active on topoisomerase II. RESULTS: The mean lethal dose 50% (LD(50)) (+/- standard deviation) of Voreloxin for primary acute myeloid leukemia blasts was 2.30 muM (+/- 1.87). Synergy experiments between Voreloxin and cytarabine identified synergism in 22 of 25 primary acute myeloid leukemia samples tested, with a mean combination index of 0.79. Apoptosis was shown to increase in a dose-dependent manner. Furthermore, Voreloxin was active in the p53-null K562 cell line suggesting that the action of Voreloxin is not affected by p53 status. The action of Voreloxin on topoisomerase II was confirmed using a DNA relaxation assay. CONCLUSIONS: Voreloxin may provide an interesting addition to the cache of drugs available for the treatment of acute myeloid leukemia, a disease with a poor long-term survival. In addition to its potent action as a single agent in dividing cells, the synergy we demonstrated between Voreloxin and cytarabine recommends further investigation of this topoisomerase II inhibitor.
Phase II multicenter trial of voreloxin as second-line therapy in chemotherapy-sensitive or refractory small cell lung cancer.[Pubmed:21252718]
J Thorac Oncol. 2011 Feb;6(2):384-6.
INTRODUCTION: Voreloxin is an anticancer quinolone derivative that intercalates DNA and inhibits topoisomerase II, causing double-strand breaks in DNA, irreversible G2 arrest, and rapid onset of apoptosis. Based on preclinical activity of Voreloxin in chemoresistant tumors, early phase I clinical activity, and a mechanism of action similar to other topoisomerase II inhibitors such as the anthracyclines and etoposide, this phase II trial was undertaken as second-line treatment of small cell lung cancer (SCLC). METHODS: Patients with extensive stage SCLC previously treated with one prior chemotherapy regimen were eligible. Patients with chemotherapy-sensitive or chemotherapy-refractory disease were considered as separate cohorts. Voreloxin (48 mg/m) was administered on the first day of each 21-day cycle for up to six cycles. The primary end point was objective response rate. RESULTS: Fifty-five patients were enrolled including 28 with refractory SCLC and 27 with sensitive SCLC; 47 were evaluable for response. Three patients with sensitive SCLC had an objective response, including one complete response and two partial responses (11% response rate based on intent to treat). No patients in the refractory cohort had a response. The primary grade 3 toxicity was neutropenia. CONCLUSION: Voreloxin has minimal activity in relapsed SCLC when administered at 48 mg/m in a 3-week schedule.


