Voreloxin HydrochlorideAntineoplastic naphthyridine analogue CAS# 175519-16-1 |
- Gatifloxacin
Catalog No.:BCC1064
CAS No.:112811-59-3
- Dexrazoxane HCl (ICRF-187, ADR-529)
Catalog No.:BCC1087
CAS No.:149003-01-0
- Doxorubicin (Adriamycin) HCl
Catalog No.:BCC1117
CAS No.:25316-40-9
- Etoposide
Catalog No.:BCC1151
CAS No.:33419-42-0
- Genistein
Catalog No.:BCN5499
CAS No.:446-72-0
Quality Control & MSDS
Number of papers citing our products

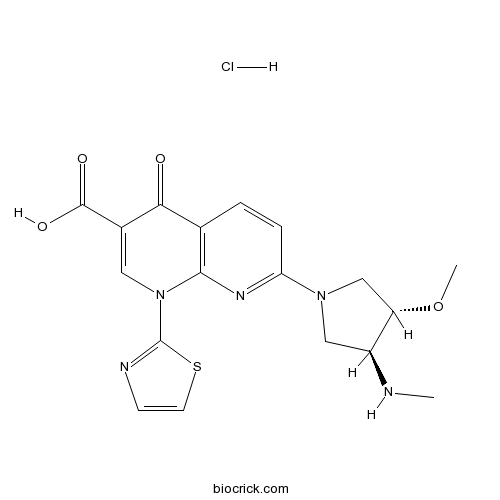
Chemical structure
3D structure
| Cas No. | 175519-16-1 | SDF | Download SDF |
| PubChem ID | 10343042 | Appearance | Powder |
| Formula | C18H20ClN5O4S | M.Wt | 437.9 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | SNS-595 Hydrochloride; Vosaroxin Hydrochloride; AG 7352 Hydrochloride | ||
| Solubility | DMSO : 2 mg/mL (4.57 mM; ultrasonic and warming and heat to 60°C) | ||
| Chemical Name | 7-[(3S,4S)-3-methoxy-4-(methylamino)pyrrolidin-1-yl]-4-oxo-1-(1,3-thiazol-2-yl)-1,8-naphthyridine-3-carboxylic acid;hydrochloride | ||
| SMILES | CNC1CN(CC1OC)C2=NC3=C(C=C2)C(=O)C(=CN3C4=NC=CS4)C(=O)O.Cl | ||
| Standard InChIKey | JJZCCQHWCOXGCL-QNTKWALQSA-N | ||
| Standard InChI | InChI=1S/C18H19N5O4S.ClH/c1-19-12-8-22(9-13(12)27-2)14-4-3-10-15(24)11(17(25)26)7-23(16(10)21-14)18-20-5-6-28-18;/h3-7,12-13,19H,8-9H2,1-2H3,(H,25,26);1H/t12-,13-;/m0./s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Voreloxin Hydrochloride is a first-in-class topoisomerase II inhibitor that intercalates DNA and induces site-selective DNA DSB, G2 arrest, and apoptosis.In Vitro:Voreloxin Hydrochloride is a first-in-class topoisomerase II poison and inhibitor that intercalates DNA and induces site-selective DNA DSB, G2 arrest, and apoptosis. Voreloxin (0.1-20 µM) inhibits topoisomerase II activity and induces site-selective DNA DSB in CCRF-CEM cells. Voreloxin (0.11, 0.33, 1, 3 µM) induces G2 arrest partially through topoisomerase II in A549 lung cancer cell line. Voreloxin cytotoxic activity requires DNA intercalation. However, Voreloxin (1-9 µM) does not generate significant levels of ROS[1]. Voreloxin has potent cytotoxic activity in AML cell lines MV4-11 and HL-60, with IC50s of 95 ± 8 nM and 884 ± 114 nM, respectively. Voreloxin in combination with cytarabine shows additive or synergistic activity in acute leukemia cell lines[2]. Voreloxin is active on the primary acute myeloid leukemia (AML) with a mean LD50 of 2.3 μM. The LD50 for voreloxin in myeloid cell lines NB4 and HL-60 is 0.59 μM ± 0.25 μM. Voreloxin causes accumulation of cells in the S and G2 phases of the cell cycle and acts on topoisomerase II[3].In Vivo:Voreloxin (20 mg/kg, i.v.) alone results in 80% reduction in bone marrow cellularity of CD-1 mice by administration one dose every 4 days repeated twice (q4d ×2). voreloxin at 10 mg/kg in combination with cytarabine causes ablation of the marrow, dilation of sinusoids, and infiltration of adipocytes in mice. Voreloxin (20 mg/kg, i.v.) combined with cytarabine causes a reversible decrease in myeloid and lymphoid cells in bone marrow and peripheral blood CD-1 mice. voreloxin (10 mg/kg, q4d ×2) and cytarabine in combination causes reversible neutropenia with a more modest impact on platelets CD-1 mice[2]. References: | |||||

Voreloxin Hydrochloride Dilution Calculator

Voreloxin Hydrochloride Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.2836 mL | 11.4181 mL | 22.8363 mL | 45.6725 mL | 57.0907 mL |
| 5 mM | 0.4567 mL | 2.2836 mL | 4.5673 mL | 9.1345 mL | 11.4181 mL |
| 10 mM | 0.2284 mL | 1.1418 mL | 2.2836 mL | 4.5673 mL | 5.7091 mL |
| 50 mM | 0.0457 mL | 0.2284 mL | 0.4567 mL | 0.9135 mL | 1.1418 mL |
| 100 mM | 0.0228 mL | 0.1142 mL | 0.2284 mL | 0.4567 mL | 0.5709 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Voreloxin is a small molecule and a naphthyridine analogue with antineoplastic activity. Vosaroxin intercalates into DNA in a site-specific manner and blocks the re-ligation process carried out by topoisomerase II during DNA replication. As a result, inhibition of DNA replication, RNA and protein synthesis occurs, followed by cell cycle arrest at G2 phase and induced p53-independent apoptosis. This agent shows a favorable toxicity profile in several aspects: it does not generate reactive oxygen species, as do anthracyclines, reducing the risk of cardiotoxicity; it is not a P-glycoprotein (P-gp) substrate, and thereby evades the common mechanism for multidrug resistance; and it has limited distribution to normal tissues and a more chemically stable molecular structure.
- Fmoc-Phe(4-Cl)-OH
Catalog No.:BCC3173
CAS No.:175453-08-4
- Fmoc-3-Pal-OH
Catalog No.:BCC2653
CAS No.:175453-07-3
- Eriosematin A
Catalog No.:BCN3465
CAS No.:175448-02-9
- Z-Phe(4-F)-OH
Catalog No.:BCC3221
CAS No.:17543-58-7
- Voreloxin
Catalog No.:BCC2044
CAS No.:175414-77-4
- S 18986
Catalog No.:BCC6081
CAS No.:175340-20-2
- Fmoc-Thr(HPO3Bzl)-OH
Catalog No.:BCC3551
CAS No.:175291-56-2
- Xanthorin
Catalog No.:BCN1122
CAS No.:17526-15-7
- O4I1
Catalog No.:BCC6542
CAS No.:175135-47-4
- NQDI 1
Catalog No.:BCC2404
CAS No.:175026-96-7
- Tonabersat
Catalog No.:BCC2009
CAS No.:175013-84-0
- Parishin C
Catalog No.:BCN3813
CAS No.:174972-80-6
- Longifloroside A
Catalog No.:BCN1123
CAS No.:175556-08-8
- Tapentadol Hydrochloride
Catalog No.:BCC9159
CAS No.:175591-09-0
- α-Conotoxin MII
Catalog No.:BCC5743
CAS No.:175735-93-0
- Lanatoside B
Catalog No.:BCN6544
CAS No.:17575-21-2
- Lanatoside C
Catalog No.:BCN6457
CAS No.:17575-22-3
- PPAHV
Catalog No.:BCC7077
CAS No.:175796-50-6
- H-Dab.HBr
Catalog No.:BCC3184
CAS No.:1758-80-1
- Wedelialactone A
Catalog No.:BCN6733
CAS No.:175862-40-5
- Valganciclovir HCl
Catalog No.:BCC4745
CAS No.:175865-59-5
- (±)-Sigmoidin A
Catalog No.:BCN3372
CAS No.:176046-04-1
- Fucosterol
Catalog No.:BCN6427
CAS No.:17605-67-3
- Zerumin A
Catalog No.:BCN3684
CAS No.:176050-48-9
Gateways to clinical trials.[Pubmed:20383346]
Methods Find Exp Clin Pharmacol. 2010 Jan-Feb;32(1):47-86.
(-)-Epigallocatechin gallate, Abafungin, ACE-031, Adapalene/benzoyl peroxide, AE-37, Aflibercept, AGS-003, Albiglutide, Alemtuzumab, Aliskiren fumarate, ALT-801, AN-2728, Anacetrapib, API, Aprepitant, ARQ-197, Ascorbic acid, Atazanavir sulfate, ATN-224, AVI-4658, Azacitidine, Azelnidipine; Belinostat, Bevacizumab, BI-2536, Biphasic insulin aspart, Bortezomib, Bovine lactoferrin, Bryostatin 1, Budesonide/formoterol fumarate; cAC10, Canfosfamide hydrochloride, Cediranib, Clofarabine, Cocaine conjugate vaccine; Darbepoetin alfa, Dasatinib, Denosumab, Disomotide, Doripenem, Dovitinib Lactate, Dronedarone hydrochloride, Drospirenone/estradiol, Dutasteride; Ecogramostim, Entinostat, Enzastaurin hydrochloride, Erlotinib hydrochloride, Everolimus, Exenatide, Ezetimibe, Ezetimibe/simvastatin; Fampridine, Fenretinide LXS, FFR-factor VIIa, Fingolimod hydrochloride, Frovatriptan; Gefitinib, Gimatecan, GP-2/GM-CSF; Iloperidone, Imatinib mesylate, Indibulin, Ipilimumab, Ivabradine hydrochloride; Lactobacillus rhamnosus, Lapatinib ditosylate, LC-07, Lenalidomide, Linifanib, Liposomal doxorubicin, Liposomal vincristine, Litenimod, Lutein; M-118, MDX-1401, MEDI-528, Midostaurin, Miglustat, MK-0657; Natalizumab, Nesiritide, NGR-TNF, Niacin/simvastatin; Obatoclax mesylate, Olaparib, Omacetaxine mepesuccinate; Paclitaxel nanoparticles, Paclitaxel-eluting stent, Palonosetron hydrochloride, Pazopanib hydrochloride, Pegfilgrastim, Pemetrexed disodium, PER.C-flu, Perifosine, PF-02341066, Pimecrolimus, Pitrakinra, Plerixafor hydrochloride, Posaconazole; Rasburicase, Recombinant human relaxin H2, ReoT3D, Retaspimycin hydrochloride, Riferminogene pecaplasmid, Rindopepimut, Romiplostim, Ronacaleret hydrochloride, Rosuvastatin calcium, Rotigotine; Sagopilone, sALP-FcD10, SAR-245409, SCH-697243, Selumetinib, Sirolimus-eluting stent, SIR-Spheres, Sitagliptin phosphate monohydrate, Sitaxentan sodium, Sorafenib, Sunitinib malate; Tadalafil, Tandutinib, Tasimelteon, Temsirolimus, Teriparatide, Tiotropium bromide, TIV, Trabectedin, Tremelimumab, TRU-016; Vadimezan, Val8-GLP-1(7-37)OH, Vandetanib, Vernakalant hydrochloride, Voreloxin, Voriconazole, Vorinostat, Yttrium 90 (90Y) ibritumomab tiuxetan; Zeaxanthin, Ziprasidone hydrochloride, Zosuquidar trihydrochloride.
Gateways to clinical trials.[Pubmed:21069103]
Methods Find Exp Clin Pharmacol. 2010 Sep;32(7):517-48.
Aclidinium bromide, AE-37, Alemtuzumab, AMA1-C1/ISA 720, Amlodipine besylate/atorvastatin calcium, Arachidonic acid, Arbaclofen placarbil, Aripiprazole, ARQ-621, Azelnidipine, Azilsartan medoxomil potassium; Bevacizumab, Biphasic insulin aspart, Bortezomib; Choriogonadotropin alfa, CTS-1027; Dapagliflozin, Dasatinib, Deforolimus, Degarelix acetate, Denufosol tetrasodium, Desvenlafaxine succinate, Dronedarone hydrochloride, Duloxetine hydrochloride, Dutasteride; Enfuvirtide, Entecavir, Etaracizumab, Everolimus, Exenatide, Ezetimibe; Ferric carboxymaltose, Fludarabine, Foretinib; Gefitinib, GFT-505, GSK-256066; HPV-6/11/16/18, HuM195/rGel, HyperAcute-Lung cancer vaccine; I5NP, Imatinib mesylate, Imexon, Insulin detemir, Insulin glargine, Ivabradine hydrochloride; L2G7, Lacosamide, Lapatinib ditosylate, Lenalidomide, Lidocaine/prilocaine, Liposomal vincristine, Liraglutide, Lixivaptan; Meningococcal (groups A, C, Y and W-135) oligosaccharide diphtheria CRM197 conjugate vaccine, Methoxy polyethylene glycol-epoetin-beta, Mirabegron, Morphine/oxycodone, MR Vaccine, MSC-1936369B, Mycophenolic acid sodium salt; Narlaprevir, N-Desmethylclozapine; Ocriplasmin, Olaparib, Olmesartan medoxomil, Olmesartan medoxomil/azelnidipine, ONO-5334, ONO-8539; Palifermin, Panitumumab, Pardoprunox hydrochloride, PCV7, Peginterferon alfa-2a, Peginterferon alfa-2b, Pemetrexed disodium, Pexelizumab, PF-337210, Pitavastatin calcium; Raltegravir potassium, Recombinant interleukin-7, Regadenoson, Reniale, Roflumilast, Rosuvastatin calcium; Safinamide mesilate, SB-1518, SCH-527123, Selumetinib, Sipuleucel-T, Solifenacin succinate, Sorafenib, Sunitinib malate; Tadalafil, Talaporfin sodium, Tanespimycin, Technosphere/Insulin, Telaprevir, Telatinib, Telcagepant, Telmisartan/hydrochlorothiazide, Teriparatide, Testosterone transdermal gel, TH-302, Tiotropium bromide, Tocilizumab, Trabedersen, Tremelimumab; Valsartan/amlodipine besylate, Vernakalant hydrochloride, Visilizumab, Voreloxin, Vorinostat.


