W-84 dibromideStabilizer of cholinergic antagonist-receptor complexes CAS# 21093-51-6 |
- AL 8697
Catalog No.:BCC8037
CAS No.:1057394-06-5
- Skepinone-L
Catalog No.:BCC1953
CAS No.:1221485-83-1
- SB202190 (FHPI)
Catalog No.:BCC1093
CAS No.:152121-30-7
- BIRB 796 (Doramapimod)
Catalog No.:BCC2535
CAS No.:285983-48-4
- TAK-715
Catalog No.:BCC3968
CAS No.:303162-79-0
- SB 203580 hydrochloride
Catalog No.:BCC4293
CAS No.:869185-85-3
Quality Control & MSDS
Number of papers citing our products

Chemical structure

3D structure
| Cas No. | 21093-51-6 | SDF | Download SDF |
| PubChem ID | 167961 | Appearance | Powder |
| Formula | C32H44Br2N4O4 | M.Wt | 708.53 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 10 mM in DMSO | ||
| Chemical Name | 3-(1,3-dioxoisoindol-2-yl)propyl-[6-[3-(1,3-dioxoisoindol-2-yl)propyl-dimethylazaniumyl]hexyl]-dimethylazanium;dibromide | ||
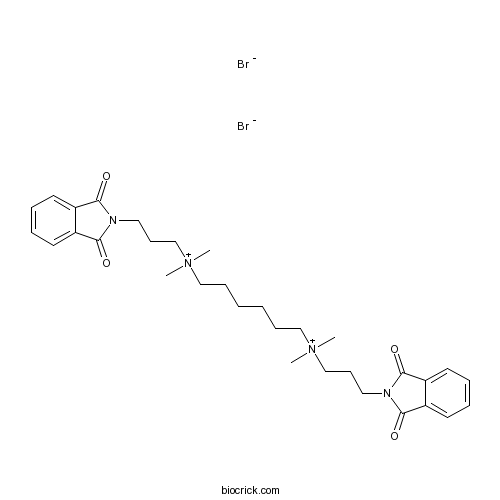
| SMILES | C[N+](C)(CCCCCC[N+](C)(C)CCCN1C(=O)C2=CC=CC=C2C1=O)CCCN3C(=O)C4=CC=CC=C4C3=O.[Br-].[Br-] | ||
| Standard InChIKey | DZRJZDQAGMZGGA-UHFFFAOYSA-L | ||
| Standard InChI | InChI=1S/C32H44N4O4.2BrH/c1-35(2,23-13-19-33-29(37)25-15-7-8-16-26(25)30(33)38)21-11-5-6-12-22-36(3,4)24-14-20-34-31(39)27-17-9-10-18-28(27)32(34)40;;/h7-10,15-18H,5-6,11-14,19-24H2,1-4H3;2*1H/q+2;;/p-2 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Stabilizes cholinergic antagonist-receptor complexes by an allosteric effect. Increases the protective effect of atropine against organophosphate poisoning. |

W-84 dibromide Dilution Calculator

W-84 dibromide Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.4114 mL | 7.0569 mL | 14.1137 mL | 28.2275 mL | 35.2843 mL |
| 5 mM | 0.2823 mL | 1.4114 mL | 2.8227 mL | 5.6455 mL | 7.0569 mL |
| 10 mM | 0.1411 mL | 0.7057 mL | 1.4114 mL | 2.8227 mL | 3.5284 mL |
| 50 mM | 0.0282 mL | 0.1411 mL | 0.2823 mL | 0.5645 mL | 0.7057 mL |
| 100 mM | 0.0141 mL | 0.0706 mL | 0.1411 mL | 0.2823 mL | 0.3528 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Org 12962 hydrochloride
Catalog No.:BCC7718
CAS No.:210821-63-9
- Sakakin
Catalog No.:BCN4916
CAS No.:21082-33-7
- CP 471474
Catalog No.:BCC2373
CAS No.:210755-45-6
- 7,8-Dihydroxy-4-Methylcoumarin
Catalog No.:BCC8290
CAS No.:2107-77-9
- 5,7-Dihydroxy-4-Methylcoumarin
Catalog No.:BCC8284
CAS No.:2107-76-8
- PD 168568 dihydrochloride
Catalog No.:BCC7702
CAS No.:210688-56-5
- 6alpha-Hydroxylycopodine
Catalog No.:BCN7403
CAS No.:21061-92-7
- 1,11b-Dihydro-11b-hydroxymaackiain
Catalog No.:BCN3914
CAS No.:210537-05-6
- 1,11b-Dihydro-11b-hydroxymedicarpin
Catalog No.:BCN3913
CAS No.:210537-04-5
- Sitaxentan sodium
Catalog No.:BCC4495
CAS No.:210421-74-2
- Odoratin-7-O-beta-D-glucopyranoside
Catalog No.:BCN8089
CAS No.:210413-47-1
- Spiradine F
Catalog No.:BCN4915
CAS No.:21040-64-2
- CART (62-76) (rat, human)
Catalog No.:BCC6008
CAS No.:210978-19-1
- BMY 7378
Catalog No.:BCC5063
CAS No.:21102-95-4
- Mahanimbine
Catalog No.:BCN3174
CAS No.:21104-28-9
- SB 265610
Catalog No.:BCC5936
CAS No.:211096-49-0
- Marsformoxide B
Catalog No.:BCN6687
CAS No.:2111-46-8
- Sobetirome
Catalog No.:BCC1957
CAS No.:211110-63-3
- Rubranol
Catalog No.:BCN4917
CAS No.:211126-61-3
- 9,17-Octadecadiene-12,14-diyne-1,11,16-triol
Catalog No.:BCN1497
CAS No.:211238-60-7
- m-Chlorophenylbiguanide hydrochloride
Catalog No.:BCC6650
CAS No.:2113-05-5
- R18
Catalog No.:BCC2383
CAS No.:211364-78-2
- Astrocasine
Catalog No.:BCN2150
CAS No.:2114-92-3
- Dendrobine
Catalog No.:BCN5923
CAS No.:2115-91-5
Effect of spacer length on the interfacial behavior of N,N'-bis(dimethylalkyl)-alpha,omega-alkanediammonium dibromide gemini surfactants in the absence and presence of ZnO nanoparticles.[Pubmed:27710822]
J Colloid Interface Sci. 2017 Jan 15;486:204-210.
In this paper the interfacial behavior of aqueous solutions of cationic gemini surfactants of the, N,N'-bis(dimethylalkyl)-alpha,omega-alkanediammoniumdibromide type (known as the 12-s-12 series), in the absence and presence of ZnO nanoparticles was studied. Equilibrium and dynamic interfacial tension between n-decane and aqueous surfactant solutions were investigated. It was concluded that the synergistic effect between surfactants and nanoparticles increases the surfactant efficiency with respect to reducing the interfacial tension. Moreover, the magnitude of the effect of ZnO nanoparticles on the interfacial tension decreases with increasing length of the spacer group in the gemini surfactant structure. Dynamic studies illustrate that the migration mechanism of gemini surfactants (regardless of the presence of ZnO) from the bulk to the interface was controlled by both diffusion and adsorption. The effect of spacer length on the contact angle and emulsion stability both with and without nanoparticles was also studied.
Dual Carbon-Bromine Stable Isotope Analysis Allows Distinguishing Transformation Pathways of Ethylene Dibromide.[Pubmed:27526716]
Environ Sci Technol. 2016 Sep 20;50(18):9855-63.
The present study investigated dual carbon-bromine isotope fractionation of the common groundwater contaminant ethylene dibromide (EDB) during chemical and biological transformations, including aerobic and anaerobic biodegradation, alkaline hydrolysis, Fenton-like degradation, debromination by Zn(0) and reduced corrinoids. Significantly different correlation of carbon and bromine isotope fractionation (LambdaC/Br) was observed not only for the processes following different transformation pathways, but also for abiotic and biotic processes with, the presumed, same formal chemical degradation mechanism. The studied processes resulted in a wide range of LambdaC/Br values: LambdaC/Br = 30.1 was observed for hydrolysis of EDB in alkaline solution; LambdaC/Br between 4.2 and 5.3 were determined for dibromoelimination pathway with reduced corrinoids and Zn(0) particles; EDB biodegradation by Ancylobacter aquaticus and Sulfurospirillum multivorans resulted in LambdaC/Br = 10.7 and 2.4, respectively; Fenton-like degradation resulted in carbon isotope fractionation only, leading to LambdaC/Br infinity. Calculated carbon apparent kinetic isotope effects ((13)C-AKIE) fell with 1.005 to 1.035 within expected ranges according to the theoretical KIE, however, biotic transformations resulted in weaker carbon isotope effects than respective abiotic transformations. Relatively large bromine isotope effects with (81)Br-AKIE of 1.0012-1.002 and 1.0021-1.004 were observed for nucleophilic substitution and dibromoelimination, respectively, and reveal so far underestimated strong bromine isotope effects.
Phosphorus-Containing Bis-allenes: Synthesis and Heterocyclization Reactions Mediated by Iodine or Copper Dibromide.[Pubmed:28357865]
Org Lett. 2017 Apr 7;19(7):1882-1885.
Bisphosphorylallenes were easily obtained in multigram scale from the Wittig-type rearrangement of bispropargyl alcohols. Unlike other conjugated bis-allenes, these reagents underwent a double cyclization mediated by iodine or copper dibromide leading to the formation of bis-1,2-oxaphospholenes.
An acridine derivative, [4,5-bis{(N-carboxy methyl imidazolium)methyl}acridine] dibromide, shows anti-TDP-43 aggregation effect in ALS disease models.[Pubmed:28000730]
Sci Rep. 2016 Dec 21;6:39490.
Amyotrophic lateral sclerosis (ALS) is a fatal neurodegenerative disease associated with aggregation of TAR DNA-binding protein-43 (TDP-43) in neuronal cells and manifests as motor neuron dysfunction &muscle atrophy. The carboxyl-terminal prion-like domain of TDP-43 can aggregate in vitro into toxic beta-sheet rich amyloid-like structures. So far, treatment options for ALS are very limited and Riluzole, which targets glutamate receptors, is the only but highly ineffective drug. Therefore, great interest exists in developing molecules for ALS treatment. Here, we have examined certain derivatives of acridine containing same side chains at position 4 &5, for inhibitory potential against TDP-43 aggregation. Among several acridine derivatives examined, AIM4, which contains polar carboxyl groups in the side arms, significantly reduces TDP-43-YFP aggregation in the powerful yeast model cell and also abolishes in vitro amyloid-like aggregation of carboxyl terminal domain of TDP-43, as observed by AFM imaging. Thus, AIM4 can be a lead molecule potentiating further therapeutic research for ALS.
Equipotent allosteric effect of W84 on [3H]NMS-binding to cardiac muscarinic receptors from guinea-pig, rat, and pig.[Pubmed:1579545]
Pharmacol Toxicol. 1992 Mar;70(3):198-200.
W84 (hexamethylene-bis-[dimethyl-(phthalimidopropyl)-ammonium bromide]) is an experimental antidote against organophosphorus poisoning and has been found to affect muscarinic cholinoceptors allosterically. The attempt was made to test whether the W84-action on muscarinic cholinoceptors depends on the species. For this purpose, the effect of W84 on the binding of [3H]N-methylscopolamine ([3H]NMS) was investigated in membrane-suspensions from the hearts of guinea pigs, rats, and pigs in 3 mM MgHPO4, 50 mM Tris, pH 7.3, at 23 degrees. W84 inhibited [3H]NMS-binding in the three membrane suspensions with similar potency (half-inhibitory concentration IC50: 2-5 microM). To evaluate the allosteric activity of W84, its effect on the dissociation of [3H]NMS was determined. At 3 microM, W84 diminished the rate of [3H]NMS-dissociation to about 20% of the control in three suspensions. At 100 microM of W84, [3H]NMS-dissociation was almost prevented. In conclusion, W84 acted equally on the cardiac cholinoceptors of guinea pigs, rats, and pigs, respectively. It can be anticipated that M2-cholinoceptors of other species would likewise be affected by W84.
Allosteric stabilization of 3H-N-methylscopolamine binding in guinea-pig myocardium by an antidote against organophosphate intoxication.[Pubmed:3054859]
Pharmacol Toxicol. 1988 Sep;63(3):163-8.
W84 (hexamethylene-bis-[dimethyl-(3-phthalimidopropyl)-ammonium bromide]) protects overadditively against an organophosphate-intoxication when applied in combination with atropine. Further experimental evidence led to the hypothesis that W84 exerted an allosteric effect on muscarinic acetylcholine receptors. In order to investigate the action of W84 on the receptor level, binding studies with 3H-N-methylscopolamine were performed in homogenized and intact guinea-pig myocardium. For sake of comparison three bispyridinium oximes were included, i.e. Uno3 (trimethylene-bis-[4-hydroxyiminomethyl-pyridinium] dibromide mono-2,6-dichlorobenzylether), obidoxime, and TMB4. In cardiac membrane suspensions, all compounds inhibited 3H-NMS-binding after 2 hrs of incubation concentration-dependently by reducing its affinity, whereas leaving the number of binding sites unaltered. However, with increasing concentrations W84 suppressed 3H-NMS-binding less than expected for a competitive antagonist. Kinetic studies revealed that W84 did not only slow the association of 3H-NMS, but additionally retarded its dissociation over the entire range of concentrations that inhibited 3H-NMS-binding. At lmM, W84 augmented the half life time of the 3H-NMS-receptor complexes from a control value of 4 min to more than 120 min. The stabilization of the radioligand-receptor complexes is indicative of an allosteric effect of W84. Obidoxime, TMB4 and Uno3 at low concentrations acted like competitive inhibitors of 3H-NMS-binding. From 10(-5)M onwards, Uno3 retarded 3H-NMS-dissociation concentration-dependently. It is concluded that the effect of bisquaternary compounds on 3H-NMS-association and -dissociation is mediated via binding to two separate sites, i.e. the muscarinic receptor site and an allosteric effector site, respectively.(ABSTRACT TRUNCATED AT 250 WORDS)
Antimuscarinic action of an alkane-bis-ammonium compound alone and in combination with (+)-benzetimide.[Pubmed:1242377]
Eur J Pharmacol. 1975 Sep-Oct;33(2):237-46.
Heptane-1,7-bis-(dimethyl-3'-phthalimidopropylammonium bromide) ('C7/3-phthalimido-propyl') in concentrations of 10(-7) to 0.5 X 10(-3) inhibited negative inotropic responses to carbachol or acetylcholine to a similar extent in the electrically stimulated isolated guinea-pig left atrium. However, the degree of antagonism of responses to carbachol was less than expected for a competitive antagonist when the higher concentrations of 'C7/3-phthalimido-propyl' were employed. When 'C7/3-phthalimido-propyl' was combined with competitive antagonists such as (+)-benzetimide, atropine or homatropine the degree of antagonism was greater than expected for combination of 2 competitive antagonists. Qualitatively similar results were obtained in the presence of practolol (1.5 X 10(-5) M). The results obtained with 'C7/3-phthalimido-propyl' are shown to agree with theoretical predictions for a metaffinoid antagonist which influences the affinities of both agonists and competitive antagonists by combining with a regulatory site distinct from, but interdependent with the binding sites for agonists and competitive antagonists. Further, it is shown that the alkane-bis-ammonium compound produces a much greater reduction in the affinity of carbachol than that of the competitive antagonists and as a consequence causes 'supra-additive' effects when combined with a competitive antagonist. The reduction in the affinity of (+)-benzetimide produced by 'C7/3-phthalimido-propyl' did not differ significantly from the reduction in the affinities of atropine or homatropine.


