WKYMVM trifluoroacetate saltSelective FPR2 and FPR3 receptor agonist CAS# 187986-11-4 |
- MK-4827
Catalog No.:BCC1761
CAS No.:1038915-60-4
- BMN-673 8R,9S
Catalog No.:BCC1422
CAS No.:1207456-00-5
- XAV-939
Catalog No.:BCC1120
CAS No.:284028-89-3
- PJ34
Catalog No.:BCC1865
CAS No.:344458-19-1
- ABT-888 (Veliparib)
Catalog No.:BCC1267
CAS No.:912444-00-9
Quality Control & MSDS
Number of papers citing our products

Chemical structure

3D structure
| Cas No. | 187986-11-4 | SDF | Download SDF |
| PubChem ID | 68661247 | Appearance | Powder |
| Formula | C41H61N9O7S2 | M.Wt | 856.11 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | W-peptide | ||
| Solubility | Soluble to 0.60 mg/ml in water | ||
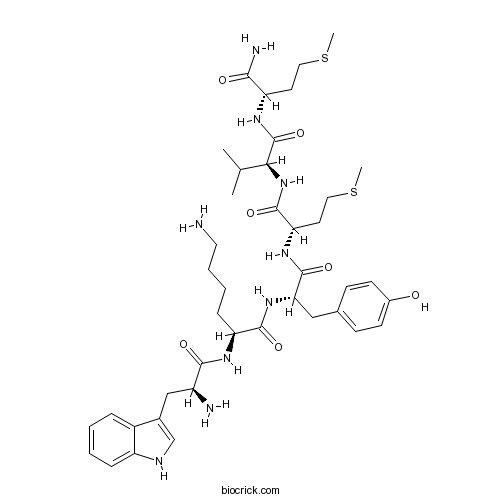
| Sequence | WKYMVM (Modifications: Met-6 = C-terminal amide) | ||
| Chemical Name | (2S)-6-amino-2-[[(2S)-2-amino-3-(1H-indol-3-yl)propanoyl]amino]-N-[(2S)-1-[[(2S)-1-[[(2S)-1-[[(2S)-1-amino-4-methylsulfanyl-1-oxobutan-2-yl]amino]-3-methyl-1-oxobutan-2-yl]amino]-4-methylsulfanyl-1-oxobutan-2-yl]amino]-3-(4-hydroxyphenyl)-1-oxopropan-2-yl]hexanamide | ||
| SMILES | CC(C)C(C(=O)NC(CCSC)C(=O)N)NC(=O)C(CCSC)NC(=O)C(CC1=CC=C(C=C1)O)NC(=O)C(CCCCN)NC(=O)C(CC2=CNC3=CC=CC=C32)N | ||
| Standard InChIKey | FMBGOORJEKQQLG-LXOXETEGSA-N | ||
| Standard InChI | InChI=1S/C41H61N9O7S2/c1-24(2)35(41(57)46-31(36(44)52)16-19-58-3)50-39(55)33(17-20-59-4)48-40(56)34(21-25-12-14-27(51)15-13-25)49-38(54)32(11-7-8-18-42)47-37(53)29(43)22-26-23-45-30-10-6-5-9-28(26)30/h5-6,9-10,12-15,23-24,29,31-35,45,51H,7-8,11,16-22,42-43H2,1-4H3,(H2,44,52)(H,46,57)(H,47,53)(H,48,56)(H,49,54)(H,50,55)/t29-,31-,32-,33-,34-,35-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Selective agonist for the formyl peptide receptors FPR2 and FPR3, expressed on immune cells. EC50 values for induction of calcium mobilization in FPR2-HL-60 cells and FPR3-HL-60 cells are 2 and 80 nM respectively. |

WKYMVM trifluoroacetate salt Dilution Calculator

WKYMVM trifluoroacetate salt Molarity Calculator

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Acetylcorynoline
Catalog No.:BCN1239
CAS No.:18797-80-3
- (+)-Corynoline
Catalog No.:BCN1235
CAS No.:18797-79-0
- Arachidonyl serotonin
Catalog No.:BCC7500
CAS No.:187947-37-1
- Serpentine
Catalog No.:BCN1162
CAS No.:18786-24-8
- BIO 1211
Catalog No.:BCC3945
CAS No.:187735-94-0
- Fmoc-Asp-OFm
Catalog No.:BCC3467
CAS No.:187671-16-5
- PNU 142633
Catalog No.:BCC7222
CAS No.:187665-65-2
- PNU 109291
Catalog No.:BCC7408
CAS No.:187665-60-7
- Tataramide B
Catalog No.:BCN3897
CAS No.:187655-56-7
- Fmoc-D-Arg(Pbf)-OH
Catalog No.:BCC3077
CAS No.:187618-60-6
- Ethyl rutinoside
Catalog No.:BCC8976
CAS No.:187539-57-7
- MaxiPost
Catalog No.:BCC7984
CAS No.:187523-35-9
- Trp-Lys-Tyr-Met-Val-Met
Catalog No.:BCC5816
CAS No.:187986-17-0
- 5-Acetyltaxachitriene A
Catalog No.:BCN7412
CAS No.:187988-48-3
- Boc-Glu-NH2
Catalog No.:BCC3387
CAS No.:18800-74-3
- Bikinin
Catalog No.:BCC5582
CAS No.:188011-69-0
- Abacavir sulfate
Catalog No.:BCC5023
CAS No.:188062-50-2
- Odoroside H
Catalog No.:BCN1163
CAS No.:18810-25-8
- AWD 131-138
Catalog No.:BCC4045
CAS No.:188116-07-6
- NocII
Catalog No.:BCC5704
CAS No.:188119-47-3
- H-Ser(tBu)-OH
Catalog No.:BCC3032
CAS No.:18822-58-7
- H-Tyr(tBu)-OH
Catalog No.:BCC3129
CAS No.:18822-59-8
- (±)-Octanoylcarnitine chloride
Catalog No.:BCC6715
CAS No.:18822-86-1
- Methylproamine
Catalog No.:BCC1741
CAS No.:188247-01-0
Effect of a novel peptide, WKYMVm- and sirolimus-coated stent on re-endothelialization and anti-restenosis.[Pubmed:26438653]
J Mater Sci Mater Med. 2015 Oct;26(10):251.
The drug-eluting stent still has limitations such as thrombosis and inflammation. These limitations often occur in the absence of endothelialization. This study investigated the effects of WKYMVm- and sirolimus-coated stents on re-endothelialization and anti-restenosis. The WKYMVm peptide, specially synthesized for homing endothelial colony-forming cells, was coated onto a bare-metal stent with hyaluronic acid through a simple dip-coating method (designated HA-Pep). Thereafter, sirolimus was consecutively coated to onto the HA-Pep (designated Pep/SRL). The cellular response to stents by human umbilical-vein endothelial cells and vascular smooth-muscle cells was examined by XTT assay. Stents were implanted into rabbit iliac arteries, isolated 6 weeks post-implantation, and then subjected to histological analysis. The peptide was well attached to the surface of the stents and the sirolimus coating made the surface smooth. The release pattern for sirolimus was similar to that of commercial sirolimus-coated stents (57.2% within 7 days, with further release for up to 28 days). Endothelial-cell proliferation was enhanced in the HA-Pep group after 7 days of culture (38.2 +/- 7.62%, compared with controls). On the other hand, the proliferation of smooth-muscle cells was inhibited in the Pep/SRL group after 7 days of culture (40.7 +/- 6.71%, compared with controls). In an animal study, the restenosis rates for the Pep/SRL group (13.5 +/- 4.50%) and commercial drug-eluting stents (Xience Prime; 9.2 +/- 7.20%) were lower than those for bare-metal stents (25.2 +/- 4.52%) and HA-Pep stents (26.9 +/- 3.88%). CD31 staining was incomplete for the bare-metal and Xience Prime groups. On the other hand, CD31 staining showed a consecutive linear pattern in the HA-Pep and Pep/SRL groups, suggesting that WKYMVm promotes endothelialization. These results indicate that the WKYMVm coating could promote endothelial healing, and consecutive coatings of WKYMVm and sirolimus onto bare-metal stents have a potential role in re-endothelialization and neointimal suppression.
Injectable PLGA microspheres encapsulating WKYMVM peptide for neovascularization.[Pubmed:26216508]
Acta Biomater. 2015 Oct;25:76-85.
UNLABELLED: Formyl peptide receptor-2 (FPR-2) is expressed in various cell types, such as phagocytes, fibroblasts, and endothelial cells. FPR-2 has been reported to play a significant role in inflammation and angiogenic response, and synthetic WKYMVm peptide has been identified as a novel peptide agonist for the FPR-2. In this study, we demonstrate that WKYMVm peptides stimulate the angiogenic potential of outgrowth endothelial cells (OECs). Upon WKYMVm peptide exposure, migration and proliferation of OECs were stimulated. WKYMVm effectively stimulated angiogenesis in tube formation assay and aortic ring assay. Furthermore, we fabricated injectable poly (lactide-co-glycolide) (PLGA) microspheres encapsulating WKYMVm peptides, which showed sustained release of cargo molecule. When WKYMVm peptide encapsulated microspheres were injected into the hind limb ischemia model, a single injection of microspheres was as effective as multiple injections of WKYMVm peptide in restoring blood flow from ischemic injury and promoting capillary growth. These results demonstrate that sustained release of WKYMVm peptide from microspheres in the application to ischemic hind limb extended angiogenic stimulation. STATEMENT OF SIGNIFICANCE: Formyl peptide receptor (FPR) has been reported to play an important role in inflammation and angiogenic response. A synthetic WKYMVm peptide has been identified as a novel peptide activating the FPR-2 that is expressed in a various cell types, such as phagocytes, fibroblasts, and endothelial cells. In this manuscript we explored a unique property of high-affinity ligand for formyl peptide receptors-2 (FPR-2) (i.e., WKYMVm). WKYMVm-induced activation of FPR2 has been reported to be crucial in host defense and inflammation by activation of phagocytes, monocytes, and lymphocytes. In this study, highlight the efficacy of WKYMVm peptide's role in inducing neovascularization in vivo hind limb ischemia model when the peptide was released from injected PLGA microspheres in sustained manner. Our results demonstrate that sustained release of WKYMVm peptide from microspheres have extended angiogenic stimulation capacity.
Biomedical therapy using synthetic WKYMVm hexapeptide.[Pubmed:27077939]
Organogenesis. 2016 Apr 2;12(2):53-60.
WKYMVm hexapeptide has been identified as a strong FPR2 agonist through a library screening of synthetic peptides. The FPR2 has been reported to play a crucial role in inflammation and angiogenic responses via stimulation of chemotaxis, migration, cell proliferation, wound healing and vessel growth. Recently, the therapeutic effects of WKYMVm have been reported in various disease models. In cutaneous wound model in diabetic mice, WKYMVm facilitated wound healing processes by stimulating the formation of capillary and arteriole and re-epithelialization. In coronary artery stenosis model, WKYMVm coating on stent promoted re-endothelialization and lowered restenosis rate. In hindlimb ischemia mouse model, intramuscular injection of WKYMVm promoted homing of exogenously transplanted endothelial colony-forming cells and neovascularization, resulting in salvaging hindlimb. Furthermore, a single injection of WKYMVm encapsulated in poly (lactide-co-glycolide) microspheres was demonstrated to be as efficient as multiple injections of WKYMVm in restoring blood flow in hindlimb ischemia model. These observations may open up promising biomedical applications of WKYMVm for tissue repairs and regenerations.
Data on the NADPH-oxidase activity induced by WKYMVm and galectin-3 in bone marrow derived and exudated neutrophils isolated from four different mouse strains.[Pubmed:28018948]
Data Brief. 2016 Dec 13;10:349-353.
Neutrophils are the key players in inflammatory reactions and the release of superoxide through the NADPH-oxidase upon neutrophil activation contributes to bacterial clearance and surrounding tissue damage. Here we describe data on the mouse neutrophil NADPH-oxidase activation induced by the mouse formyl peptide receptor (Fpr) agonist WKYMVm and galectin-3. Neutrophils isolated from bone marrow, peritoneal exudated, and in vitro TNFalpha primed bone marrow neutrophils from four different laboratory strains (C57BL/6, DBA/1, BALB/c and NMRI) were used. Both Fpr agonist and galectin-3 activated neutrophils to release superoxide. No differences were observed in the amounts of superoxide released from neutrophils derived from four different strains.
Phagocyte activation by Trp-Lys-Tyr-Met-Val-Met, acting through FPRL1/LXA4R, is not affected by lipoxin A4.[Pubmed:12410796]
Scand J Immunol. 2002 Nov;56(5):470-6.
Lipoxin A4 (LXA4) has been shown to bind to the leucocyte formyl peptide receptor (FPR) homologue, FPRL1, without triggering the biological activities induced by other FPRL1 agonists. We investigated the direct effect of LXA4 as well as the effect on agonist-induced biological responses using transfected HL-60 cells expressing FPR, FPRL1 or FPRL2. LXA4 neither induced an intracellular rise in calcium in these transfectants nor affected the response induced by the peptide Trp-Lys-Tyr-Met-Val-Met (WKYMVM), an agonist that activates cells through FPRL1 and -2. Both agonists induced Erk-2 activation; however, the eicosanoid-induced activity was independent of FPRL1 and FPRL2. Moreover, LXA4 was unable to trigger neutrophil upregulation of complement receptor 3 and respiratory burst, and it had no effect on the responses induced by triggering with WKYMVM. We conclude that LXA4 is unable to affect the WKYMVM-induced signalling through FPRL1 and suggest that it acts through a receptor different from FPRL1.
The synthetic peptide Trp-Lys-Tyr-Met-Val-Met-NH2 specifically activates neutrophils through FPRL1/lipoxin A4 receptors and is an agonist for the orphan monocyte-expressed chemoattractant receptor FPRL2.[Pubmed:11285256]
J Biol Chem. 2001 Jun 15;276(24):21585-93.
Neutrophils express the G protein-coupled N-formyl peptide receptor (FPR) and its homologue FPRL1, whereas monocytes express FPR, FPRL1, and FPRL2, an orphan receptor sharing 83% amino acid identity with FPRL1. FPRL1 is a promiscuous receptor activated by serum amyloid A and by different synthetic peptides, including the hexapeptide Trp-Lys-Tyr-Met-Val-d-Met-NH(2) (WKYMVm). By measuring calcium flux in HL-60 cells transfected with FPR, FPRL1, or FPRL2, we show that WKYMVm activated all three receptors, whereas the l-conformer WKYMVM activated exclusively FPRL1 and FPRL2. The functionality of FPRL2 was further assessed by the ability of HL-60-FPRL2 cells to migrate toward nanomolar concentrations of hexapeptides. The half-maximal effective concentrations of WKYMVM for calcium mobilization in HL-60-FPRL1 and HL-60-FPRL2 cells were 2 and 80 nm, respectively. Those of WKYMVm were 75 pm and 3 nm. The tritiated peptide WK[3,5-(3)H(2)]YMVM bound to FPRL1 (K(D) approximately 160 nm), but not to FPR. The two conformers similarly inhibited binding of (125)I-labeled WKYMVm to FPRL2-expressing cells (IC(50) approximately 2.5-3 micrometer). Metabolic labeling with orthophosphoric acid revealed that FPRL1 was differentially phosphorylated upon addition of the l- or d-conformer, indicating that it induced different conformational changes. In contrast to FPRL1, FPRL2 was already phosphorylated in the absence of agonist and not evenly distributed in the plasma membrane of unstimulated cells. However, both receptors were internalized upon addition of either of the two conformers. Taken together, the results indicate that neutrophils are activated by WKYMVM through FPRL1 and that FPRL2 is a chemotactic receptor transducing signals in myeloid cells.
Identification of the peptides that stimulate the phosphoinositide hydrolysis in lymphocyte cell lines from peptide libraries.[Pubmed:8626507]
J Biol Chem. 1996 Apr 5;271(14):8170-5.
Peptides which stimulate the formation of inositol phosphates (InoPs) in lymphocyte cell lines were identified by screening synthetic peptide libraries composed of random sequences of hexapeptides. The peptides containing the consensus sequence XKYX(P/V)M were found to be most active in the phospholipase C (PLC)-mediated formation of InoPs in a human B myeloma cell line, U266. The peptides also stimulated the phosphoinositide hydrolysis and the release of [Ca2+]i in HL60 and U937 cell lines. On the other hand, these peptides showed no effect in the following cell lines: NIH3T3, PC12, Daudi, Sp2, Jurkat, H9, Molt-4, SupT-1, K562, and RBL-2H3. The result suggests the possibility that the peptides may have cell type specificity. Experiments with one of the active peptides, WKYMVM-NH2 showed that its action mimics the effect of AlF4- which is a G-protein activator in the InoPs generation, and pertussis toxin partially blocked the InoPs accumulation and [Ca2+]i release induced by the peptide in the U266 cells. Binding assays with the peptide labeled with 125I showed that U266 cells have a saturable number of binding sites for the peptide. Taken together, these results suggest that the peptides could activate PLC-mediated signal transduction via a pertussis toxin-sensitive G-protein coupled receptor in certain cell types.


