Withaferin ACAS# 5119-48-2 |
- MK-4827
Catalog No.:BCC1761
CAS No.:1038915-60-4
- BMN-673 8R,9S
Catalog No.:BCC1422
CAS No.:1207456-00-5
- XAV-939
Catalog No.:BCC1120
CAS No.:284028-89-3
- PJ34
Catalog No.:BCC1865
CAS No.:344458-19-1
- ABT-888 (Veliparib)
Catalog No.:BCC1267
CAS No.:912444-00-9
Quality Control & MSDS
Number of papers citing our products

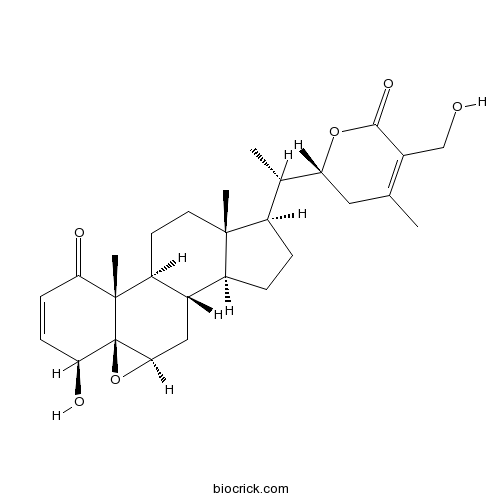
Chemical structure

3D structure
| Cas No. | 5119-48-2 | SDF | Download SDF |
| PubChem ID | 265237 | Appearance | White powder |
| Formula | C28H38O6 | M.Wt | 470.6 |
| Type of Compound | Steroids | Storage | Desiccate at -20°C |
| Synonyms | Withaferine | ||
| Solubility | DMSO : 100 mg/mL (212.49 mM; Need ultrasonic) | ||
| SMILES | CC1=C(C(=O)OC(C1)C(C)C2CCC3C2(CCC4C3CC5C6(C4(C(=O)C=CC6O)C)O5)C)CO | ||
| Standard InChIKey | DBRXOUCRJQVYJQ-CKNDUULBSA-N | ||
| Standard InChI | InChI=1S/C28H38O6/c1-14-11-21(33-25(32)17(14)13-29)15(2)18-5-6-19-16-12-24-28(34-24)23(31)8-7-22(30)27(28,4)20(16)9-10-26(18,19)3/h7-8,15-16,18-21,23-24,29,31H,5-6,9-13H2,1-4H3/t15-,16-,18+,19-,20-,21+,23-,24+,26+,27-,28+/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Withaferin A(WFA) has anti-inflammatory, anti-oxidant, immune modulatory, cardioactive, antithrombotic and central nervous system effects. 2. Withaferin A is a potent breast cancer anti-metastatic agent and the anti-metastatic activity of WFA is, at least in part, mediated through its effects on vimentin and vimentin ser56 phosphorylation. 3. Withaferin A induces p53-dependent apoptosis by repression of HPV oncogenes and upregulation of tumor suppressor proteins in human cervical cancer cells, it can be exploited as a potent therapeutic agent for the treatment and prevention of cervical cancer without deleterious effects. 4. Withaferin A could act as an anti-fibrotic compound against fibroproliferative diseases, including, but not limited to, cardiac interstitial fibrosis. 5. Withaferin A sensitizes TRAIL-induced apoptosis through reactive oxygen species-mediated up-regulation of death receptor 5 and down-regulation of c-FLIP. 6. Withaferin A is a proteasomal inhibitor promotes healing after injury and exerts anabolic effect on osteoporotic bone. |
| Targets | ROS | NF-kB | p53 | STAT | PARP | Bcl-2/Bax | Caspase | TGF-β/Smad | TNF-α | COX | PGE | PI3K | Akt | JNK | p38MAPK |

Withaferin A Dilution Calculator

Withaferin A Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.1249 mL | 10.6247 mL | 21.2495 mL | 42.4989 mL | 53.1237 mL |
| 5 mM | 0.425 mL | 2.1249 mL | 4.2499 mL | 8.4998 mL | 10.6247 mL |
| 10 mM | 0.2125 mL | 1.0625 mL | 2.1249 mL | 4.2499 mL | 5.3124 mL |
| 50 mM | 0.0425 mL | 0.2125 mL | 0.425 mL | 0.85 mL | 1.0625 mL |
| 100 mM | 0.0212 mL | 0.1062 mL | 0.2125 mL | 0.425 mL | 0.5312 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Boc-D-Asp(OBzl)-OH
Catalog No.:BCC3371
CAS No.:51186-58-4
- Episyringaresinol
Catalog No.:BCN7023
CAS No.:51152-20-6
- (R)-(-)-Ibuprofen
Catalog No.:BCC4062
CAS No.:51146-57-7
- (S)-(+)-Ibuprofen
Catalog No.:BCC4042
CAS No.:51146-56-6
- (+)-trans-Isolimonene
Catalog No.:BCC9236
CAS No.:5113-87-1
- Cyclic Pifithrin-α hydrobromide
Catalog No.:BCC2407
CAS No.:511296-88-1
- 8-Bromo-cGMP, sodium salt
Catalog No.:BCC6935
CAS No.:51116-01-9
- Gitogenin
Catalog No.:BCN3886
CAS No.:511-96-6
- Plumieride
Catalog No.:BCN5631
CAS No.:511-89-7
- Vitamin D4
Catalog No.:BCC2042
CAS No.:511-28-4
- Totarol
Catalog No.:BCN4627
CAS No.:511-15-9
- Sugiol
Catalog No.:BCN3164
CAS No.:511-05-7
- Diosgenin
Catalog No.:BCN6272
CAS No.:512-04-9
- Yamogenin
Catalog No.:BCN8277
CAS No.:512-06-1
- Raffinose
Catalog No.:BCN8427
CAS No.:512-69-6
- Ascaridole
Catalog No.:BCC8121
CAS No.:512-85-6
- Boc-Arg(Z)-OH
Catalog No.:BCC3068
CAS No.:51219-18-2
- 6,8-Diprenylgenistein
Catalog No.:BCN4805
CAS No.:51225-28-6
- Wighteone
Catalog No.:BCN5632
CAS No.:51225-30-0
- N-Phthaloyl-Phe-OH
Catalog No.:BCC3016
CAS No.:5123-55-7
- Amsacrine
Catalog No.:BCC4309
CAS No.:51264-14-3
- Gallocatechin gallate
Catalog No.:BCN6803
CAS No.:5127-64-0
- 2,6-Dimethyl-3,7-octadiene-2,6-diol
Catalog No.:BCN5633
CAS No.:51276-34-7
- Afzelechin 3-O-xyloside
Catalog No.:BCN7774
CAS No.:512781-45-2
Withaferin A induces p53-dependent apoptosis by repression of HPV oncogenes and upregulation of tumor suppressor proteins in human cervical cancer cells.[Pubmed:21859835]
Carcinogenesis. 2011 Nov;32(11):1697-705.
Cervical cancer is caused by human papilloma virus (HPV) expressing E6 and E7 oncoproteins, which are known to inactivate tumor suppressor proteins p53 and pRb, respectively. Repression of HPV oncoproteins would therefore result in reactivation of tumor suppressor pathways and cause apoptosis in cancer cells. Withaferin A (WA), the active component of the medicinal plant Withania Somnifera, has exhibited inhibitory effects against several different cancers. We examined the activity of WA on human cervical cancer cells in vitro and in vivo. WA potently inhibited proliferation of the cervical cancer cells, CaSki (IC(50) 0.45 +/- 0.05 muM). Mechanistically, WA was found to (i) downregulate expression of HPV E6 and E7 oncoproteins, (ii) induce accumulation of p53, (iii) increase levels of p21(cip1/waf1) and its interaction with proliferating cell nuclear antigen (PCNA), (iv) cause G(2)/M cell cycle arrest, associated with modulation of cyclin B1, p34(cdc2) and PCNA levels, (v) decrease the levels of STAT3 and its phosphorylation at Tyr(705) and Ser(727) and (vi) alter expression levels of p53-mediated apoptotic markers-Bcl2, Bax, caspase-3 and cleaved PARP. In vivo, WA resulted in reduction of nearly 70% of the tumor volume in athymic nude mice with essentially similar trend in the modulation of molecular markers as in vitro. This is the first demonstration indicating that WA significantly downregulates expression of HPV E6/E7 oncogenes and restores the p53 pathway, resulting in apoptosis of cervical cancer cells. Together, our data suggest that WA can be exploited as a potent therapeutic agent for the treatment and prevention of cervical cancer without deleterious effects.
Antiplatelet, anticoagulant, and profibrinolytic activities of withaferin A.[Pubmed:24534482]
Vascul Pharmacol. 2014 Mar;60(3):120-6.
Withaferin A (WFA), an active compound from Withania somnifera, is widely researched for its anti-inflammatory, cardioactive and central nervous system effects. However, antiplatelet, anticoagulant, and profibrinolytic properties of WFA have not been studied. In this study, the anticoagulant activities of WFA were measured by monitoring activated partial thromboplastin-time (aPTT), prothrombin time (PT), fibrin polymerization, platelet aggregation, thrombus formation, and the activities of cell-based thrombin and activated factor X (FXa). The effects of WFA on the expressions of plasminogen activator inhibitor type 1 (PAI-1) and tissue-type plasminogen activator (t-PA) were also tested in tumor necrosis factor-alpha (TNF-alpha) activated human umbilical vein endothelial cells (HUVECs). Our data showed that WFA inhibited thrombin-catalyzed fibrin polymerization and platelet aggregation, FeCl3-induced thrombus formation, prolonged aPTT and PT significantly and inhibited the activities and production of thrombin and FXa. WFA prolonged in vivo and ex vivo bleeding time and inhibited TNF-alpha induced PAI-1 production. Furthermore, PAI-1/t-PA ratio was significantly decreased by WFA. Collectively, these results indicate that WFA possesses antithrombotic activities and suggest that the current study could provide bases for the development of new anticoagulant agents.
Withaferin A inhibits breast cancer invasion and metastasis at sub-cytotoxic doses by inducing vimentin disassembly and serine 56 phosphorylation.[Pubmed:21538350]
Int J Cancer. 2011 Dec 1;129(11):2744-55.
Withaferin A (WFA) is purified from the plant Withania somnifera and inhibits the vimentin cytoskeleton. Vimentin overexpression in cancer correlates with metastatic disease, induction of epithelial to mesenchymal transition and reduced patient survival. As vimentin functions in cell motility, we wanted to test the hypothesis that WFA inhibits cancer metastasis by disrupting vimentin function. These data showed that WFA had weak cytotoxic and apoptotic activity at concentrations less than or equal to 500 nM, but retained potent anti-invasive activity at these low doses. Imaging of breast cancer cell lines revealed that WFA induces perinuclear vimentin accumulation followed by rapid vimentin depolymerization. A concomitant induction of vimentin ser56 phosphorylation was observed, which is consistent with vimentin disassembly. Structure activity relationships were established using a set of chemically modified WFA analogs and showed that the predicted vimentin-binding region of WFA is necessary to induce vimentin ser56 phosphorylation and for its anti-invasive activity. Pharmacokinetic studies in mice revealed that WFA reaches peak concentrations up to 2 muM in plasma with a half-life of 1.36 hr following a single 4 mg/kg dose. In a breast cancer metastasis mouse model, WFA showed dose-dependent inhibition of metastatic lung nodules and induced vimentin ser56 phosphorylation, with minimal toxicity to lung tissue. Based upon these studies, we conclude that WFA is a potent breast cancer anti-metastatic agent and the anti-metastatic activity of WFA is, at least in part, mediated through its effects on vimentin and vimentin ser56 phosphorylation.
Withaferin-A reduces type I collagen expression in vitro and inhibits development of myocardial fibrosis in vivo.[Pubmed:22900077]
PLoS One. 2012;7(8):e42989.
Type I collagen is the most abundant protein in the human body. Its excessive synthesis results in fibrosis of various organs. Fibrosis is a major medical problem without an existing cure. Excessive synthesis of type I collagen in fibrosis is primarily due to stabilization of collagen mRNAs. We recently reported that intermediate filaments composed of vimentin regulate collagen synthesis by stabilizing collagen mRNAs. Vimentin is a primary target of Withaferin-A (WF-A). Therefore, we hypothesized that WF-A may reduce type I collagen production by disrupting vimentin filaments and decreasing the stability of collagen mRNAs. This study is to determine if WF-A exhibits anti-fibrotic properties in vitro and in vivo and to elucidate the molecular mechanisms of its action. In lung, skin and heart fibroblasts WF-A disrupted vimentin filaments at concentrations of 0.5-1.5 microM and reduced 3 fold the half-lives of collagen alpha1(I) and alpha2(I) mRNAs and protein expression. In addition, WF-A inhibited TGF-beta1 induced phosphorylation of TGF-beta1 receptor I, Smad3 phosphorylation and transcription of collagen genes. WF-A also inhibited in vitro activation of primary hepatic stellate cells and decreased their type I collagen expression. In mice, administration of 4 mg/kg WF-A daily for 2 weeks reduced isoproterenol-induced myocardial fibrosis by 50%. Our findings provide strong evidence that Withaferin-A could act as an anti-fibrotic compound against fibroproliferative diseases, including, but not limited to, cardiac interstitial fibrosis.
Withaferin A sensitizes TRAIL-induced apoptosis through reactive oxygen species-mediated up-regulation of death receptor 5 and down-regulation of c-FLIP.[Pubmed:19345731]
Free Radic Biol Med. 2009 Jun 15;46(12):1639-49.
Withaferin A (Wit A) has reportedly shown cytotoxicity in a variety of tumor cell lines. Here, we show that cotreatment with subtoxic doses of Wit A and tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) induces apoptosis in human renal cancer cells, Caki cells, but not in human normal mesangial cells. Moreover, the combined treatment with Wit A and TRAIL dramatically induces apoptosis in various cancer cell types, suggesting that this combined treatment might offer an attractive strategy for safely treating human cancers. Treatment of Caki cells with Wit A up-regulated death receptor 5 (DR5) in a C/EBP homologous protein (CHOP)-dependent manner. Interestingly, a Wit A-induced increase in ROS levels preceded the up-regulation of CHOP and DR5. The involvement of ROS in CHOP-mediated DR5 up-regulation was confirmed by the result that pretreatment with an antioxidant, NAC or catalase, inhibited Wit A-induced up-regulation of both CHOP and DR5. We also found that Wit A treatment down-regulated c-FLIP via NF-kappaB-mediated transcriptional control as well as ROS signaling pathways. Taken together, our results show that DR5 up-regulation and c-FLIP down-regulation contribute to the sensitizing effect of Wit A on TRAIL-mediated apoptosis in cancer cells.
Production of reactive oxygen species by withaferin A causes loss of type collagen expression and COX-2 expression through the PI3K/Akt, p38, and JNK pathways in rabbit articular chondrocytes.[Pubmed:24016823]
Exp Cell Res. 2013 Nov 1;319(18):2822-34.
Withaferin A (WFA) is a major chemical constituent of Withania somnifera, also known as Indian ginseng. Many recent reports have provided evidence of its anti-tumor, anti-inflammation, anti-oxidant, and immune modulatory activities. Although the compound appears to have a large number of effects, its defined mechanisms of action have not yet been determined. We investigated the effects of WFA on loss of type collagen expression and inflammation in rabbit articular chondrocytes. WFA increased the production of reactive oxygen species, suggesting the induction of oxidative stress, in a dose-dependent manner. Also, we confirmed that WFA causes loss of type collagen expression and inflammation as determined by a decrease of type II collagen expression and an increase of cyclooxygenase-2 (COX-2) expression via western blot analysis in a dose- and time- dependent manner. WFA also reduced the synthesis of sulfated proteoglycan via Alcian blue staining and caused the synthesis of prostaglandin E2 (PGE2) via assay kit in dose- and time-dependent manners. Treatment with N-acetyl-L-cysteine (NAC), an antioxidant, inhibited WFA-induced loss of type II collagen expression and increase in COX-2 expression, accompanied by inhibition of reactive oxygen species production. WFA increased phosphorylation of both Akt and p38. Inhibition of PI3K/Akt, p38, and JNK with LY294002 (LY), SB203580 (SB), or SP600125 (SP) in WFA-treated cells rescued the expression of type II collagen and suppressed the expression of COX-2. These results demonstrate that WFA induces loss of type collagen expression and inflammation via PI3K/Akt, p38, and JNK by generating reactive oxygen species in rabbit articular chondrocytes.
Withaferin a strongly elicits IkappaB kinase beta hyperphosphorylation concomitant with potent inhibition of its kinase activity.[Pubmed:17150968]
J Biol Chem. 2007 Feb 16;282(7):4253-64.
The transcription factor NFkappaB plays a critical role in normal and pathophysiological immune responses. Therefore, NFkappaB and the signaling pathways that regulate its activation have become a major focus of drug development programs. Withania somnifera (WS) is a medicinal plant that is widely used in Palestine for the treatment of various inflammatory disorders. In this study we show that the leave extract of WS, as well as its major constituent Withaferin A (WA), potently inhibits NFkappaB activation by preventing the tumor necrosis factor-induced activation of IkappaB kinase beta via a thioalkylation-sensitive redox mechanism, whereas other WS-derived steroidal lactones, such as withanolide A and 12-deoxywithastramonolide, are far less effective. To our knowledge, this is the first communication of IkappaB kinase beta inhibition by a plant-derived inhibitor, coinciding with MEK1/ERK-dependent Ser-181 hyperphosphorylation. This prevents IkappaB phosphorylation and degradation, which subsequently blocks NFkappaB translocation, NFkappaB/DNA binding, and gene transcription. Taken together, our results indicate that pure WA or WA-enriched WS extracts can be considered as a novel class of NFkappaB inhibitors, which hold promise as novel anti-inflammatory agents for treatment of various inflammatory disorders and/or cancer.
The tumor proteasome is a primary target for the natural anticancer compound Withaferin A isolated from "Indian winter cherry".[Pubmed:17093135]
Mol Pharmacol. 2007 Feb;71(2):426-37.
Withaferin A (WA) is a steroidal lactone purified from medicinal plant "Indian Winter Cherry" that is widely researched for its variety of properties, including antitumor effects. However, the primary molecular target of WA is unknown. By chemical structure analysis, we hypothesized that Withaferin A might be a natural proteasome inhibitor. Computational modeling studies consistently predict that C1 and C24 of WA are highly susceptible toward a nucleophilic attack by the hydroxyl group of N-terminal threonine of the proteasomal chymotrypsin subunit beta5. Furthermore, WA potently inhibits the chymotrypsin-like activity of a purified rabbit 20S proteasome (IC50=4.5 microM) and 26S proteasome in human prostate cancer cultures (at 5-10 microM) and xenografts (4-8 mg/kg/day). Inhibition of prostate tumor cellular proteasome activity in cultures and in vivo by WA results in accumulation of ubiquitinated proteins and three proteasome target proteins (Bax, p27, and IkappaB-alpha) accompanied by androgen receptor protein suppression (in androgen-dependent LNCaP cells) and apoptosis induction. Treatment of WA under conditions of the aromatic ketone reduction, or reduced form of Celastrol, had significantly decreased the proteasome-inhibitory and apoptosis-inducing activities. Treatment of human prostate PC-3 xenografts with WA for 24 days resulted in 70% inhibition of tumor growth in nude mice, associated with 56% inhibition of the tumor tissue proteasomal chymotrypsinlike activity. Our results demonstrate that the tumor proteasome beta5 subunit is the primary target of WA, and inhibition of the proteasomal chymotrypsin-like activity by WA in vivo is responsible for, or contributes to, the antitumor effect of this ancient medicinal compound.
Withaferin A is a potent inhibitor of angiogenesis.[Pubmed:15516832]
Angiogenesis. 2004;7(2):115-22.
The medicinal plant Withania somnifera is widely researched for its anti-inflammatory, cardioactive and central nervous system effects. In Ayurveda , the major Traditional Indian medicine system, extracts from W. somnifera are distinctively employed for the treatment of arthritis and menstrual disorders. Because these conditions involve angiogenic processes we hypothesized that the W. somnifera extracts might contain angiogenesis inhibitors. We employed an endothelial cell-sprouting assay to monitor the purification of substances from W. somnifera root extracts and isolated as the active principle the previously known natural product Withaferin A. We show that Withaferin A inhibits human umbilical vein endothelial cell (HUVEC) sprouting in three-dimensional collagen-I matrix at doses which are relevant to NF-kappa B-inhibitory activity. Withaferin A inhibits cell proliferation in HUVECs (IC50 =12 nM) at doses that are significantly lower than those required for tumor cell lines through a process associated with inhibition of cyclin D1 expression. We propose that the inhibition of NF-kappa B by Withaferin A in HUVECs occurs by interference with the ubiquitin-mediated proteasome pathway as suggested by the increased levels of poly-ubiquitinated proteins. Finally, Withaferin A is shown to exert potent anti-angiogenic activity in vivo at doses that are 500-fold lower than those previously reported to exert anti-tumor activity in vivo. In conclusion, our findings identify a novel mode of action of Withaferin A, which highlights the potential use of this natural product for cancer treatment or prevention.


