XMD8-92BMK1/ERK5 inhibitor,highly selective CAS# 1234480-50-2 |
Quality Control & MSDS
Number of papers citing our products

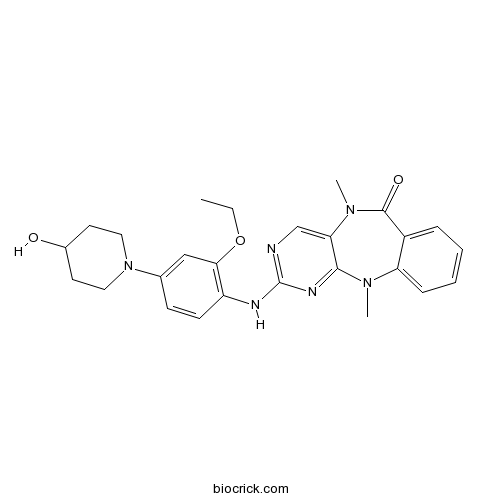
Chemical structure
3D structure
| Cas No. | 1234480-50-2 | SDF | Download SDF |
| PubChem ID | 46843772 | Appearance | Powder |
| Formula | C26H30N6O3 | M.Wt | 474.57 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : 50 mg/mL (105.36 mM; Need ultrasonic) | ||
| Chemical Name | 2-[2-ethoxy-4-(4-hydroxypiperidin-1-yl)anilino]-5,11-dimethylpyrimido[4,5-b][1,4]benzodiazepin-6-one | ||
| SMILES | CCOC1=C(C=CC(=C1)N2CCC(CC2)O)NC3=NC=C4C(=N3)N(C5=CC=CC=C5C(=O)N4C)C | ||
| Standard InChIKey | QAPAJIZPZGWAND-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C26H30N6O3/c1-4-35-23-15-17(32-13-11-18(33)12-14-32)9-10-20(23)28-26-27-16-22-24(29-26)30(2)21-8-6-5-7-19(21)25(34)31(22)3/h5-10,15-16,18,33H,4,11-14H2,1-3H3,(H,27,28,29) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | ERK5 (BMK1) and BRD4 inhibitor (Kd values are 80 and 190 nM, respectively). Also inhibits DCAMKL2, PLK4 and TNK1 (Kd values are 190, 600 and 890 nM). Blocks growth factor-induced activation of cellular BMK1 and reduces BMK1 activity in in vitro kinase assays. Also reduces BMK1-dependent transactivating activity of MEF2C. Inhibits proliferation in a variety of cancer cell lines; blocks tumor cell proliferation and tumor-associated angiogenesis. |

XMD8-92 Dilution Calculator

XMD8-92 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.1072 mL | 10.5359 mL | 21.0717 mL | 42.1434 mL | 52.6793 mL |
| 5 mM | 0.4214 mL | 2.1072 mL | 4.2143 mL | 8.4287 mL | 10.5359 mL |
| 10 mM | 0.2107 mL | 1.0536 mL | 2.1072 mL | 4.2143 mL | 5.2679 mL |
| 50 mM | 0.0421 mL | 0.2107 mL | 0.4214 mL | 0.8429 mL | 1.0536 mL |
| 100 mM | 0.0211 mL | 0.1054 mL | 0.2107 mL | 0.4214 mL | 0.5268 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
IC50: XMD8-92 has been synthesized as a potent inhibitor of Mitogen-activated protein kinase 7 (MAPK7/BMK1; Kd = 80 nM). XMD8-92 blocks EGF-induced activation of BMK1 with IC50 of 240 nM [1].
The mitogen-activated protein kinases (MAPKs) are crucial components of signaling cascades that regulate numerous physiological processes. Four MAPK pathways have been identified thus far, including extracelluar-signal-regulated kinase 1/2 (ERK1/2), c-Jun-amino-terminal kinase (JNK), p38, and BMK1. XMD8-92 is a MAPKs kinase inhibitor with anti-cancer activity against lung and cervical cancers.
In vitro: In a previous study, XMD8-92 was shown to inhibit AsPC-1 cancer cell proliferation and tumor xenograft growth. In XMD8-92 treated tumors, significant downregulation of DCLK1was found and several of its downstream targets, including c-MYC, KRAS, NOTCH1, ZEB1, ZEB2, SNAIL, SLUG, OCT4, SOX2, NANOG, KLF4, LIN28, VEGFR1, and VEGFR2) via upregulation of tumor suppressor miRNAs, such as let-7a, miR-144, miR-200a-c, and miR-143/145. XMD8-92 was, however, not found to affect BMK1 downstream genes p21 and p53. These findings suggested that XMD8-92 treatment led to the inhibition of DCLK1 and downstream oncogenic pathways, which would be a promising chemotherapeutic agent against PDAC [2].
In vivo: In both immunocompetent and immunodeficient mice, XMD8-92 treatment was found to able to block the growth of lung and cervical xenograft tumors, respectively, by 95%. This remarkable anti-tumor effect of XMD8-92 in lung and cervical xenograft tumor models was due to its capacity to inhibit tumor cell proliferation through the PML suppressioninducted p21 checkpoint protein, as well as by blocking of the contribution of BMK1 in tumorassociated angiogenesis [3].
Clinical trial: XMD8-92 is still at preclinical development stage up to this point.
Reference:
[1] Yang Q, Lee JD. Targeting the BMK1 MAP kinase pathway in cancer therapy. Clin Cancer Res. 2011;17(11):3527-32.
[2] Sureban SM, May R, Weygant N, Qu D, Chandrakesan P, Bannerman-Menson E, Ali N, Pantazis P, Westphalen CB, Wang TC, Houchen CW. XMD8-92 inhibits pancreatic tumor xenograft growth via a DCLK1-dependent mechanism. Cancer Lett. 2014;351(1):151-61.
[3] Yang Q, Deng X, Lu B, Cameron M, Fearns C, Patricelli MP, et al. Pharmacological inhibition of
BMK1 suppresses tumor growth through promyelocytic leukemia protein. Cancer Cell. 2010;18:258–67.
- Rivastigmine
Catalog No.:BCC1900
CAS No.:123441-03-2
- Boc-Glu(Ofm)-OH
Catalog No.:BCC3391
CAS No.:123417-18-5
- LY2606368
Catalog No.:BCC4105
CAS No.:1234015-52-1
- Z-Arg-OH
Catalog No.:BCC3060
CAS No.:1234-35-1
- ELR510444
Catalog No.:BCC6418
CAS No.:1233948-35-0
- Methyl 4-caffeoylquinate
Catalog No.:BCN3442
CAS No.:123372-74-7
- CAY10650
Catalog No.:BCC4178
CAS No.:1233706-88-1
- kb NB 142-70
Catalog No.:BCC1675
CAS No.:1233533-04-4
- Dipsacobioside
Catalog No.:BCN6552
CAS No.:123350-57-2
- AZ20
Catalog No.:BCC1389
CAS No.:1233339-22-4
- Clofarabine
Catalog No.:BCC1078
CAS No.:123318-82-1
- 2'-O-Methylhelichrysetin
Catalog No.:BCN4792
CAS No.:123316-64-3
- LRRK2-IN-1
Catalog No.:BCC1706
CAS No.:1234480-84-2
- PM 102
Catalog No.:BCC6105
CAS No.:1234564-95-4
- PNU 282987
Catalog No.:BCC7318
CAS No.:123464-89-1
- LY2608204
Catalog No.:BCC4969
CAS No.:1234703-40-2
- SAR191801
Catalog No.:BCC6393
CAS No.:1234708-04-3
- 3-O-Caffeoylquinic acid methyl ester
Catalog No.:BCN3484
CAS No.:123483-19-2
- Demethylmurrayanine
Catalog No.:BCN4721
CAS No.:123497-84-7
- Biperiden HCl
Catalog No.:BCC4565
CAS No.:1235-82-1
- Linderaspirone A
Catalog No.:BCN6122
CAS No.:1235126-46-1
- Azelnidipine
Catalog No.:BCC4400
CAS No.:123524-52-7
- AQ-RA 741
Catalog No.:BCC7314
CAS No.:123548-16-3
- rac BHFF
Catalog No.:BCC7644
CAS No.:123557-91-5
XMD8-92 inhibits pancreatic tumor xenograft growth via a DCLK1-dependent mechanism.[Pubmed:24880079]
Cancer Lett. 2014 Aug 28;351(1):151-61.
XMD8-92 is a kinase inhibitor with anti-cancer activity against lung and cervical cancers, but its effect on pancreatic ductal adenocarcinoma (PDAC) remains unknown. Doublecortin-like kinase1 (DCLK1) is upregulated in various cancers including PDAC. In this study, we showed that XMD8-92 inhibits AsPC-1 cancer cell proliferation and tumor xenograft growth. XMD8-92 treated tumors demonstrated significant downregulation of DCLK1 and several of its downstream targets (including c-MYC, KRAS, NOTCH1, ZEB1, ZEB2, SNAIL, SLUG, OCT4, SOX2, NANOG, KLF4, LIN28, VEGFR1, and VEGFR2) via upregulation of tumor suppressor miRNAs let-7a, miR-144, miR-200a-c, and miR-143/145; it did not however affect BMK1 downstream genes p21 and p53. These data taken together suggest that XMD8-92 treatment results in inhibition of DCLK1 and downstream oncogenic pathways (EMT, pluripotency, angiogenesis and anti-apoptotic), and is a promising chemotherapeutic agent against PDAC.
ERK5 kinase activity is dispensable for cellular immune response and proliferation.[Pubmed:27679845]
Proc Natl Acad Sci U S A. 2016 Oct 18;113(42):11865-11870.
Unlike other members of the MAPK family, ERK5 contains a large C-terminal domain with transcriptional activation capability in addition to an N-terminal canonical kinase domain. Genetic deletion of ERK5 is embryonic lethal, and tissue-restricted deletions have profound effects on erythroid development, cardiac function, and neurogenesis. In addition, depletion of ERK5 is antiinflammatory and antitumorigenic. Small molecule inhibition of ERK5 has been shown to have promising activity in cell and animal models of inflammation and oncology. Here we report the synthesis and biological characterization of potent, selective ERK5 inhibitors. In contrast to both genetic depletion/deletion of ERK5 and inhibition with previously reported compounds, inhibition of the kinase with the most selective of the new inhibitors had no antiinflammatory or antiproliferative activity. The source of efficacy in previously reported ERK5 inhibitors is shown to be off-target activity on bromodomains, conserved protein modules involved in recognition of acetyl-lysine residues during transcriptional processes. It is likely that phenotypes reported from genetic deletion or depletion of ERK5 arise from removal of a noncatalytic function of ERK5. The newly reported inhibitors should be useful in determining which of the many reported phenotypes are due to kinase activity and delineate which can be pharmacologically targeted.
Canonical and kinase activity-independent mechanisms for extracellular signal-regulated kinase 5 (ERK5) nuclear translocation require dissociation of Hsp90 from the ERK5-Cdc37 complex.[Pubmed:23428871]
Mol Cell Biol. 2013 Apr;33(8):1671-86.
The mitogen-activated protein (MAP) kinase extracellular signal-regulated kinase 5 (ERK5) plays a crucial role in cell proliferation, regulating gene transcription. ERK5 has a unique C-terminal tail which contains a transcriptional activation domain, and activates transcription by phosphorylating transcription factors and acting itself as a transcriptional coactivator. However, the molecular mechanisms that regulate its nucleocytoplasmatic traffic are unknown. We have used tandem affinity purification to identify proteins that interact with ERK5. We show that ERK5 interacts with the Hsp90-Cdc37 chaperone in resting cells, and that inhibition of Hsp90 or Cdc37 results in ERK5 ubiquitylation and proteasomal degradation. Interestingly, activation of cellular ERK5 induces Hsp90 dissociation from the ERK5-Cdc37 complex, leading to ERK5 nuclear translocation and activation of transcription, by a mechanism which requires the autophosphorylation at its C-terminal tail. Consequently, active ERK5 is no longer sensitive to Hsp90 or Cdc37 inhibitors. Cdc37 overexpression also induces Hsp90 dissociation and the nuclear translocation of a kinase-inactive form of ERK5 which retains transcriptional activity. This is the first example showing that ERK5 transcriptional activity does not require kinase activity. Since Cdc37 cooperates with ERK5 to promote cell proliferation, Cdc37 overexpression (as happens in some cancers) might represent a new, noncanonical mechanism by which ERK5 regulates tumor proliferation.
Discovery of a benzo[e]pyrimido-[5,4-b][1,4]diazepin-6(11H)-one as a Potent and Selective Inhibitor of Big MAP Kinase 1.[Pubmed:21412406]
ACS Med Chem Lett. 2011 Mar 10;2(3):195-200.
Kinome-wide selectivity profiling of a collection of 2-amino-pyrido[2,3-d]pyrimidines followed by cellular structure-activity relationship-guided optimization resulted in the identification of moderately potent and selective inhibitors of BMK1/ERK5 exemplified by 11, 18, and 21. For example, 11 possesses a dissociation constant (K(d)) for BMK1 of 19 nM, a cellular IC(50) for inhibiting epidermal growth factor induced BMK1 autophosphorylation of 0.19 +/- 0.04 muM, and an Ambit KINOMEscan selectivity score (S(5)) of 0.035. Inhibitors 18 and 21 are also potent BMK1 inhibitors and possess favorable pharmacokinetic properties which enable their use as pharmacological probes of BMK1-dependent phenomena as well as starting points for further optimization efforts.
Targeting the BMK1 MAP kinase pathway in cancer therapy.[Pubmed:21385929]
Clin Cancer Res. 2011 Jun 1;17(11):3527-32.
The big mitogen activated protein kinase 1 (BMK1) pathway is the most recently discovered and least-studied mammalian mitogen-activated protein (MAP) kinase cascade, ubiquitously expressed in all types of cancer cells tested so far. Mitogens and oncogenic signals strongly activate this cellular MAP kinase pathway, thereby passing down proliferative, survival, chemoresistance, invasive, and angiogenic signals in tumor cells. Recently, several pharmacologic small molecule inhibitors of this pathway have been developed. Among them, the BMK1 inhibitor XMD8-92 blocks cellular BMK1 activation and significantly suppresses tumor growth in lung and cervical tumor models and is well tolerated in animals. On the other hand, MEK5 inhibitors, BIX02188, BIX02189, and compound 6, suppress cellular MEK5 activity, but no data exist to date on their effectiveness in animals.
Pharmacological inhibition of BMK1 suppresses tumor growth through promyelocytic leukemia protein.[Pubmed:20832753]
Cancer Cell. 2010 Sep 14;18(3):258-67.
BMK1 is activated by mitogens and oncogenic signals and, thus, is strongly implicated in tumorigenesis. We found that BMK1 interacted with promyelocytic leukemia protein (PML), and inhibited its tumor-suppressor function through phosphorylation. Furthermore, activated BMK1 notably inhibited PML-dependent activation of p21. To further investigate the BMK-mediated inhibition of the tumor suppressor activity of PML in tumor cells, we developed a small-molecule inhibitor of the kinase activity of BMK1, XMD8-92. Inhibition of BMK1 by XMD8-92 blocked tumor cell proliferation in vitro and significantly inhibited tumor growth in vivo by 95%, demonstrating the efficacy and tolerability of BMK1-targeted cancer treatment in animals.


