AQ-RA 741M2 antagonist,selective and high affinity CAS# 123548-16-3 |
- BCX 1470
Catalog No.:BCC1413
CAS No.:217099-43-9
- A 205804
Catalog No.:BCC3944
CAS No.:251992-66-2
- A 286982
Catalog No.:BCC3946
CAS No.:280749-17-9
- AEBSF.HCl
Catalog No.:BCC1219
CAS No.:30827-99-7
- Nafamostat
Catalog No.:BCC4187
CAS No.:81525-10-2
- Nafamostat Mesylate(FUT-175)
Catalog No.:BCC1228
CAS No.:82956-11-4
Quality Control & MSDS
Number of papers citing our products

Chemical structure

3D structure
| Cas No. | 123548-16-3 | SDF | Download SDF |
| PubChem ID | 129989 | Appearance | Powder |
| Formula | C27H37N5O2 | M.Wt | 463.62 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 100 mM in 1eq. HCl and to 100 mM in DMSO and to 100 mM in ethanol | ||
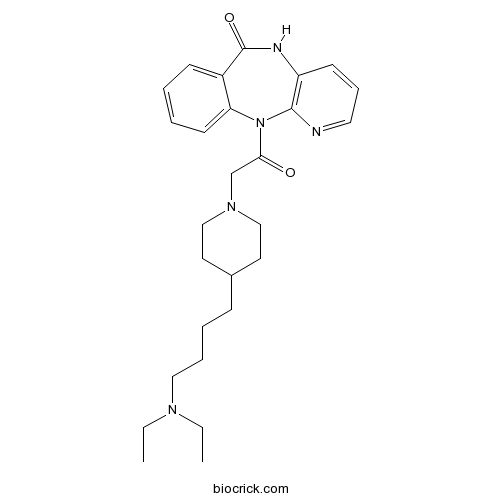
| Chemical Name | 11-[2-[4-[4-(diethylamino)butyl]piperidin-1-yl]acetyl]-5H-pyrido[2,3-b][1,4]benzodiazepin-6-one | ||
| SMILES | CCN(CC)CCCCC1CCN(CC1)CC(=O)N2C3=CC=CC=C3C(=O)NC4=C2N=CC=C4 | ||
| Standard InChIKey | BCUGCHZRMKTPMU-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C27H37N5O2/c1-3-30(4-2)17-8-7-10-21-14-18-31(19-15-21)20-25(33)32-24-13-6-5-11-22(24)27(34)29-23-12-9-16-28-26(23)32/h5-6,9,11-13,16,21H,3-4,7-8,10,14-15,17-20H2,1-2H3,(H,29,34) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | High affinity, selective muscarinic M2 receptor antagonist (pKi values are 8.3, 7.7 and 6.82 for M2, M1 and M3 receptors, respectively). Displays cardioselectivity in vivo, over intestinal, tracheal and bladder muscarinic receptors; inhibits vagally and agonist-induced bradycardia. |

AQ-RA 741 Dilution Calculator

AQ-RA 741 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.1569 mL | 10.7847 mL | 21.5694 mL | 43.1388 mL | 53.9235 mL |
| 5 mM | 0.4314 mL | 2.1569 mL | 4.3139 mL | 8.6278 mL | 10.7847 mL |
| 10 mM | 0.2157 mL | 1.0785 mL | 2.1569 mL | 4.3139 mL | 5.3923 mL |
| 50 mM | 0.0431 mL | 0.2157 mL | 0.4314 mL | 0.8628 mL | 1.0785 mL |
| 100 mM | 0.0216 mL | 0.1078 mL | 0.2157 mL | 0.4314 mL | 0.5392 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
AQ-RA 741 is a potent and selective M2 antagonist, with high affinity for cardiac M2 sites (pKi = 8.30) [1].
The M2 muscarinic receptor subtype is involved in the regulation of heart rate, mediating muscarinic receptor-dependent movement, antinociceptive responses and temperature control [2].
In radioligand binding studies, the affinity of AQ-RA 741 for cardiac M2 sites, cortical M1 sites and grandular M3 sites are of pKi values of 8.30, 7.70 and 6.82, respectively. That means AQ-RA 741 showed high affinity for cardiac M2 sites, compared to that for cortical M1 sites and grandular M3 sites. Functional studies showed that AQ-RA 741 is a competitive antagonist. It has a 60 to 87-fold higher affinity to bind cardiac muscarinic receptors than to bind muscarinic receptors in tracheal, intestinal or bladder smooth muscle [1].
M2 selectivity of AQ-RA 741 was also confirmed by in vivo experiments. In rats, guinea-pigs and cats, vagally or agonist-induced bradycardia (?log ID50 = 7.24–7.53 i.v.) were preferentially inhibited by AQ-RA 741. The ratio range of observed potencies between effects mediated by cardiac and other muscarinic receptor was between 9- and greater than 100-fold. These results concluded that AQ-RA 741 is of remarkable in vivo selectivity as a potent and selective M2 antagonist [1].
References:
[1]. Doods H, Entzeroth M and Mayer N. Cardioselectivity of AQ-RA 741, a novel tricyclic antimuscarinic drug. Eur J Pharmacol, 1991, 192(1):147-52.
[2]. Gomeza J, Shannon H, Kostenis E, et al. Pronounced pharmacologic deficits in M2 muscarinic acetylcholine receptor knockout mice. Proc Natl Acad Sci U S A, 1999, 96(4):1692-7.
- Azelnidipine
Catalog No.:BCC4400
CAS No.:123524-52-7
- Linderaspirone A
Catalog No.:BCN6122
CAS No.:1235126-46-1
- Biperiden HCl
Catalog No.:BCC4565
CAS No.:1235-82-1
- Demethylmurrayanine
Catalog No.:BCN4721
CAS No.:123497-84-7
- 3-O-Caffeoylquinic acid methyl ester
Catalog No.:BCN3484
CAS No.:123483-19-2
- SAR191801
Catalog No.:BCC6393
CAS No.:1234708-04-3
- LY2608204
Catalog No.:BCC4969
CAS No.:1234703-40-2
- PNU 282987
Catalog No.:BCC7318
CAS No.:123464-89-1
- PM 102
Catalog No.:BCC6105
CAS No.:1234564-95-4
- LRRK2-IN-1
Catalog No.:BCC1706
CAS No.:1234480-84-2
- XMD8-92
Catalog No.:BCC2062
CAS No.:1234480-50-2
- Rivastigmine
Catalog No.:BCC1900
CAS No.:123441-03-2
- rac BHFF
Catalog No.:BCC7644
CAS No.:123557-91-5
- Peptide YY(3-36), PYY, human
Catalog No.:BCC1041
CAS No.:123583-37-9
- Curcumadionol
Catalog No.:BCN3561
CAS No.:1235984-45-8
- PHP 501 trifluoroacetate
Catalog No.:BCC6193
CAS No.:1236105-75-1
- 740 Y-P
Catalog No.:BCC5861
CAS No.:1236188-16-1
- Clerosterol glucoside
Catalog No.:BCN6123
CAS No.:123621-00-1
- Salviaplebeiaside
Catalog No.:BCN7304
CAS No.:1236273-88-3
- Diosbulbin L
Catalog No.:BCN7305
CAS No.:1236285-87-2
- Tenofovir maleate
Catalog No.:BCC4262
CAS No.:1236287-04-9
- Fmoc-Glu(OBzl)-OH
Catalog No.:BCC3493
CAS No.:123639-61-2
- NS 398
Catalog No.:BCC6857
CAS No.:123653-11-2
- Iguratimod
Catalog No.:BCC1641
CAS No.:123663-49-0
Cardioselectivity of AQ-RA 741, a novel tricyclic antimuscarinic drug.[Pubmed:2040358]
Eur J Pharmacol. 1991 Jan 3;192(1):147-52.
The interaction of the AF-DX 116 analogue, AQ-RA 741 (11-[[4-[4-(diethylamino)butyl]-1-piperidinyl]acetyl]-5,11- dihydro-6H-pyrido[2,3-b] [1,4]benzodiazepin-6-one), with muscarinic receptors, in vitro and in vivo, was examined. In radioligand binding studies, AQ-RA 741 showed high affinity for cardiac M2 sites (pKi = 8.30), intermediate affinity for cortical M1 sites (pKi = 7.70) and low affinity for glandular M3 sites (pKi = 6.82). Functional studies showed AQ-RA 741 to be a competitive antagonist and to have a 60 to 87-fold higher affinity for cardiac muscarinic receptors than for muscarinic receptors in intestinal, tracheal or bladder smooth muscle. In vivo experiments confirmed the M2 selectivity of AQ-RA 741. In rats, cats and guinea-pigs AQ-RA 741 preferentially inhibited the vagally or agonist-induced bradycardia (-log ID50 = 7.24-7.53 i.v.). The ratio of potencies observed between effects mediated by cardiac and other muscarinic receptor ranged between 9- and more than 100-fold. The results show that AQ-RA 741 is a potent and selective M2 antagonist with remarkable in vivo selectivity.
Pharmacological profile of selective muscarinic receptor antagonists on guinea-pig ileal smooth muscle.[Pubmed:8200421]
Eur J Pharmacol. 1994 Mar 3;253(3):275-81.
The present study examined the effects of a series of tricyclic muscarinic receptor antagonists on muscarinic receptors present in the guinea-pig ileum, both in vitro and in vivo. The selectivity profiles of these antagonists and that of atropine were determined by their affinity for cortical muscarinic M1, cardiac M2 and submandibular M3 receptors and for m4 receptors expressed in CHO cells. The compounds pirenzepine, UH-AH 37, AQ-RA 391 and AQ-RA 618 possessed high affinity (pKi 7.94-8.22) for muscarinic M1 receptors. Pirenzepine exhibited the most pronounced muscarinic M1 selectivity. AF-DX 384 and AQ-RA 741 possessed an approximately 10-fold higher affinity for the cardiac muscarinic M2 receptor than AF-DX 116. However, both compounds also exhibited high affinity for muscarinic m4 receptors. High affinity for muscarinic M3 and m4 receptors was observed for UH-AH 37, AQ-RA 391 and AQ-RA 681. The antagonists were then tested for their interaction with the muscarinic receptors which are responsible for the methacholine-induced contraction of longitudinal muscle in vitro, circular muscle in vivo and muscarinic receptors which mediate the distension-evoked ascending reflex contraction of circular muscle in vitro. Compounds showing high affinity for muscarinic M3 receptors (e.g. AQ-RA 618) were the most potent antagonists in the functional experiments. Comparison of the binding displacement data with the functional results indicates that the effects of methacholine on the longitudinal and circular muscle of the guinea-pig ileum were predominantly mediated by muscarinic M3-type receptors. In contrast, the correlation between muscarinic M2 receptor affinity and antagonism of muscarinic receptors in the ileum was very weak.
Antagonist binding profiles of five cloned human muscarinic receptor subtypes.[Pubmed:1994002]
J Pharmacol Exp Ther. 1991 Feb;256(2):727-33.
A variety of muscarinic antagonists are currently used as tools to pharmacologically subclassify muscarinic receptors into M1, M2 and M3 subtypes. In the present study, we have determined the affinity profiles of several of these antagonists at five cloned human muscarinic receptors (m1-m5) stably expressed in Chinese hamster ovary cells (CHO-K1). At all five receptors, the (R)-enantiomers of trihexyphenidyl and hexbutinol displayed considerably higher affinities (up to 525-fold) than their corresponding (S)-isomers. The stereoselectivity ratios [inhibition constant(S)/inhibition constant(R)] for both pairs of enantiomers were lowest at m2 receptors, suggesting that less stringent configurational demands are made by this receptor subtype. The "M1-selective" antagonist (R)-trihexyphenidyl displayed high affinities for m1 and m4 receptors. The "M2-selective" antagonists himbacine, (+-)-5,11-dihydro-11- ([(2-[(dipropylamino)methyl]-1- piperidinyl)ethyl)amino]carbonyl)-6H-pyrido(2,3-b)(1,4)benzodiazepine-6- one (AF-DX 384), 11-[4-[4-(diethylamino)butyl]-1-piperidinyl)acetyl)-5,11- dihydro-6H-pyrido(2,3-b) (1,4)benzodiazepine-6-one (AQ-RA 741) and (+)-(11-[2-[(diethylamino) methyl]-1-piperidinyl)acetyl)-5,11-di-hydro-6H-pyrido(2,3-b)(1,4) benzodiazepine-6-one [AF-DX 250; the (+)-enantiomer of AF-DX 116] exhibited high affinities for m2 and m4, intermediate affinities for m1 and m3 and low affinities for m5 receptors. This selectivity profile was most prominent for AQ-RA 741, which displayed 195- and 129-fold higher affinities for m2 and m4 receptors than for m5 receptors.(ABSTRACT TRUNCATED AT 250 WORDS)


