2-ThioUTP tetrasodium saltPotent and selective P2Y2 agonist CAS# 1343364-70-4 |
- ABT-751 (E7010)
Catalog No.:BCC1085
CAS No.:141430-65-1
- CHM 1
Catalog No.:BCC2387
CAS No.:154554-41-3
- Podophyllotoxin
Catalog No.:BCN5957
CAS No.:518-28-5
- D-64131
Catalog No.:BCC1510
CAS No.:74588-78-6
- CYT997 (Lexibulin)
Catalog No.:BCC4601
CAS No.:917111-44-5
- MPC 6827 hydrochloride
Catalog No.:BCC8040
CAS No.:917369-31-4
Quality Control & MSDS
Number of papers citing our products

Chemical structure

3D structure
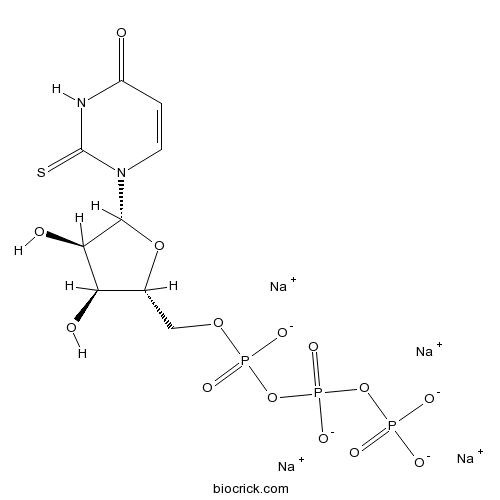
| Cas No. | 1343364-70-4 | SDF | Download SDF |
| PubChem ID | 54591573 | Appearance | Powder |
| Formula | C9H11N2Na4O14P3S | M.Wt | 588.13 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in water (supplied pre-dissolved at a concentration of 10mM) | ||
| Chemical Name | tetrasodium;[[[(2R,3S,4R,5R)-3,4-dihydroxy-5-(4-oxo-2-sulfanylidenepyrimidin-1-yl)oxolan-2-yl]methoxy-oxidophosphoryl]oxy-oxidophosphoryl] phosphate | ||
| SMILES | C1=CN(C(=S)NC1=O)C2C(C(C(O2)COP(=O)([O-])OP(=O)([O-])OP(=O)([O-])[O-])O)O.[Na+].[Na+].[Na+].[Na+] | ||
| Standard InChIKey | QCBCNKBBEOQRRB-ODQFIEKDSA-J | ||
| Standard InChI | InChI=1S/C9H15N2O14P3S.4Na/c12-5-1-2-11(9(29)10-5)8-7(14)6(13)4(23-8)3-22-27(18,19)25-28(20,21)24-26(15,16)17;;;;/h1-2,4,6-8,13-14H,3H2,(H,18,19)(H,20,21)(H,10,12,29)(H2,15,16,17);;;;/q;4*+1/p-4/t4-,6-,7-,8-;;;;/m1..../s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Potent and selective P2Y2 agonist (EC50 values are 0.035, 0.35 and 1.5 μM for hP2Y2, hP2Y4 and hP2Y6 receptors respectively). |

2-ThioUTP tetrasodium salt Dilution Calculator

2-ThioUTP tetrasodium salt Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.7003 mL | 8.5015 mL | 17.003 mL | 34.0061 mL | 42.5076 mL |
| 5 mM | 0.3401 mL | 1.7003 mL | 3.4006 mL | 6.8012 mL | 8.5015 mL |
| 10 mM | 0.17 mL | 0.8502 mL | 1.7003 mL | 3.4006 mL | 4.2508 mL |
| 50 mM | 0.034 mL | 0.17 mL | 0.3401 mL | 0.6801 mL | 0.8502 mL |
| 100 mM | 0.017 mL | 0.085 mL | 0.17 mL | 0.3401 mL | 0.4251 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- alpha,beta-Methyleneadenosine 5'-triphosphate trisodium salt
Catalog No.:BCC7603
CAS No.:1343364-54-4
- Tolcapone
Catalog No.:BCC2334
CAS No.:134308-13-7
- BIMU 8
Catalog No.:BCC7928
CAS No.:134296-40-5
- 3,5-Dibromo-4-[3-(dimethylamino)propoxy]cinnamic acid
Catalog No.:BCN1582
CAS No.:134276-56-5
- Daphnelantoxin B
Catalog No.:BCN3228
CAS No.:134273-12-4
- RKI-1447
Catalog No.:BCC1903
CAS No.:1342278-01-6
- Methylcobalamin
Catalog No.:BCC5188
CAS No.:13422-55-4
- Fmoc-Tyr(PO3Bzl2)-OH
Catalog No.:BCC3566
CAS No.:134150-51-9
- INCB8761(PF-4136309)
Catalog No.:BCC1649
CAS No.:1341224-83-6
- TP-0903
Catalog No.:BCC6462
CAS No.:1341200-45-0
- Ponasterone A
Catalog No.:BCN6184
CAS No.:13408-56-5
- (S)-4-Carboxyphenylglycine
Catalog No.:BCC6603
CAS No.:134052-73-6
- Epoxymicheliolide
Catalog No.:BCN8275
CAS No.:1343403-10-0
- Ro 0437626
Catalog No.:BCC7276
CAS No.:134362-79-1
- Epoxomicin
Catalog No.:BCC1235
CAS No.:134381-21-8
- Seocalcitol
Catalog No.:BCC1944
CAS No.:134404-52-7
- Dehydroandrographolide
Catalog No.:BCN1260
CAS No.:134418-28-3
- CA 074
Catalog No.:BCC1141
CAS No.:134448-10-5
- Discodermide
Catalog No.:BCN1834
CAS No.:134458-00-7
- BW-B 70C
Catalog No.:BCC7013
CAS No.:134470-38-5
- Richenoic acid
Catalog No.:BCN6185
CAS No.:134476-74-7
- Trimethylvinylammonium(1+)
Catalog No.:BCN1820
CAS No.:13448-18-5
- 1-Cinnamoyl-3-hydroxypyrrolidine
Catalog No.:BCN6497
CAS No.:1344876-77-2
- Gardenoin J
Catalog No.:BCN7666
CAS No.:1345109-46-7
Conventional high-performance liquid chromatography versus derivative spectrophotometry for the determination of 1,3,6-pyrenetrisulfonic acid trisodium salt and 1,3,6,8-pyrenetetrasulfonic acid tetrasodium salt in the color additive D&C Green No. 8 (Pyranine).[Pubmed:24315677]
J Chromatogr A. 2014 Jan 10;1324:238-41.
Specifications in the U.S. Code of Federal Regulations for the color additive D&C Green No. 8 (Colour Index No. 59040) limit the levels of the subsidiary colors 1,3,6-pyrenetrisulfonic acid trisodium salt (P3S) and 1,3,6,8-pyrenetetrasulfonic acid tetrasodium salt (P4S). The present paper describes a comparative study of two possible methods to replace the currently used multi-step TLC/spectrophotometry method of separating and quantifying the minor components P3S and P4S in G8. One of the new approaches uses conventional high-performance liquid chromatography (HPLC) and the other, derivative spectrophotometry. While the derivative spectrophotometric method was shown to be inadequate for the analysis of minor components overwhelmed by components of much higher concentration, the HPLC method was proven highly effective. The closely related, very polar compounds P3S and P4S were separated by the new HPLC method in less than 4 min using a conventional HPLC instrument. P3S and P4S were quantified by using five-point calibration curves with data points that ranged from 0.45 to 7.63% and from 0.13 to 1.82%, by weight, for P3S and P4S, respectively. The HPLC method was applied to the analysis of test portions from 20 batches of D&C Green No. 8 submitted to the U.S. Food and Drug Administration for certification.
Intermolecular interaction of nickel (ii) phthalocyanine tetrasulfonic acid tetrasodium salt with bovine serum albumin: A multi-technique study.[Pubmed:27831822]
Nucleosides Nucleotides Nucleic Acids. 2017 Feb;36(2):122-138.
The interaction of nickel (II) phthalocyanine tetrasulfonic acid tetrasodium salt with bovine serum albumin (BSA) has been investigated by combination of fluorescence, UV-vis absorption, Fourier transform infrared (FT-IR), and circular dichorism (CD) spectroscopies as well as through molecular docking. Fluorescence quenching and absorption spectra were investigated as a mean for estimating the binding parameters. Analysis of fluorescence quenching data at different temperatures was performed in order to specify the thermodynamics parameters for interactions of phthalocyanine complex with BSA. According to experimental data it was suggested that phthalocyanine had a significant binding affinity to BSA and the process was entropy driven. Based on the results of molecular docking it was indicated that the main active binding site for this phthalocyanine complex is site I in subdomain IIA of BSA. The results provide useful information for understanding the binding mechanism of anticancer drug-albumin and gives insight into the biological activity and metabolism of the drug in blood.
Meso-tetrakis(p-sulfonatophenyl)N-confused porphyrin tetrasodium salt: a potential sensitizer for photodynamic therapy.[Pubmed:22582931]
J Med Chem. 2012 Jun 14;55(11):5110-20.
A water-soluble derivative of N-confused porphyrin (NCP) was synthesized, and the photodynamic therapeutic (PDT) application was investigated by photophysical and in vitro studies. High singlet oxygen quantum yield in water at longer wavelength and promising IC(50) values in a panel of cancer cell lines ensure the potential candidacy of the sensitizer as a PDT drug. Reactive oxygen species (ROS) generation on PDT in MDA-MB 231 cells and the apoptotic pathway of cell death was illustrated using different techniques.
NF546 [4,4'-(carbonylbis(imino-3,1-phenylene-carbonylimino-3,1-(4-methyl-phenylene)-car bonylimino))-bis(1,3-xylene-alpha,alpha'-diphosphonic acid) tetrasodium salt] is a non-nucleotide P2Y11 agonist and stimulates release of interleukin-8 from human monocyte-derived dendritic cells.[Pubmed:19815812]
J Pharmacol Exp Ther. 2010 Jan;332(1):238-47.
The G protein-coupled P2Y(11) receptor is involved in immune system modulation. In-depth physiological evaluation is hampered, however, by a lack of selective and potent ligands. By screening a library of sulfonic and phosphonic acid derivatives at P2Y(11) receptors recombinantly expressed in human 1321N1 astrocytoma cells (calcium and cAMP assays), the selective non-nucleotide P2Y(11) agonist NF546 [4,4'-(carbonylbis(imino-3,1-phenylene-carbonylimino-3,1-(4-methyl-phenylene)carb onylimino))-bis(1,3-xylene-alpha,alpha'-diphosphonic acid) tetrasodium salt] was identified. NF546 had a pEC(50) of 6.27 and is relatively selective for P2Y(11) over P2Y(1), P2Y(2), P2Y(4), P2Y(6), P2Y(12), P2X(1), P2X(2), and P2X(2)-X(3). Adenosine-5'-O-(3-thio)triphosphate (ATPgammaS), a nonhydrolyzable analog of the physiological P2Y(11) agonist ATP, and NF546 use a common binding site as suggested by molecular modeling studies and their competitive behavior toward the nanomolar potency antagonist NF340 [4,4'-(carbonylbis(imino-3,1-(4-methyl-phenylene)carbonylimino))bis(naphthalene-2 ,6-disulfonic acid) tetrasodium salt] in Schild analysis. The pA(2) of NF340 was 8.02 against ATPgammaS and 8.04 against NF546 (calcium assays). NF546 was further tested for P2Y(11)-mediated effects in monocyte-derived dendritic cells. Similarly to ATPgammaS, NF546 led to thrombospondin-1 secretion and inhibition of lipopolysaccharide-stimulated interleukin-12 release, whereas NF340 inhibited these effects. Further, for the first time, it was shown that ATPgammaS or NF546 stimulation promotes interleukin 8 (IL-8) release from dendritic cells, which could be inhibited by NF340. In conclusion, we have described the first selective, non-nucleotide agonist NF546 for P2Y(11) receptors in both recombinant and physiological expression systems and could show a P2Y(11)-stimulated IL-8 release, further supporting the immunomodulatory role of P2Y(11) receptors.
Synthesis and potency of novel uracil nucleotides and derivatives as P2Y2 and P2Y6 receptor agonists.[Pubmed:18514530]
Bioorg Med Chem. 2008 Jun 15;16(12):6319-32.
The phosphate, uracil, and ribose moieties of uracil nucleotides were varied structurally for evaluation of agonist activity at the human P2Y(2), P2Y(4), and P2Y(6) receptors. The 2-thio modification, found previously to enhance P2Y(2) receptor potency, could be combined with other favorable modifications to produce novel molecules that exhibit high potencies and receptor selectivities. Phosphonomethylene bridges introduced for stability in analogues of UDP, UTP, and uracil dinucleotides markedly reduced potency. Truncation of dinucleotide agonists of the P2Y(2) receptor, in the form of Up(4)-sugars, indicated that a terminal uracil ring is not essential for moderate potency at this receptor and that specific SAR patterns are observed at this distal end of the molecule. Key compounds reported in this study include 9, alpha,beta-methylene-UDP, a P2Y(6) receptor agonist; 30, Up(4)-phenyl ester and 34, Up(4)-[1]glucose, selective P2Y(2) receptor agonists; dihalomethylene phosphonate analogues 16 and 41, selective P2Y(2) receptor agonists; 43, the 2-thio analogue of INS37217 (P(1)-(uridine-5')-P(4)-(2'-deoxycytidine-5')tetraphosphate), a potent and selective P2Y(2) receptor agonist.
Synthesis and structure-activity relationships of uracil nucleotide derivatives and analogues as agonists at human P2Y2, P2Y4, and P2Y6 receptors.[Pubmed:17125260]
J Med Chem. 2006 Nov 30;49(24):7076-87.
A series of UTP, UDP, and UMP derivatives and analogues were synthesized and evaluated at the human pyrimidinergic P2Y receptor subtypes P2Y2, P2Y4, and P2Y6 stably expressed in 1321N1 astrocytoma cells. Substituents at N3 of UTP were poorly tolerated by P2Y2 and P2Y4 receptors. In contrast, a large phenacyl substituent at N3 of UDP was well tolerated by the P2Y6 receptor, yielding a potent and selective P2Y6 receptor agonist (3-phenacyl-UDP, EC50=70 nM, >500-fold selective). The most potent and selective P2Y2 receptor agonist of the present series was 2-thio-UTP (EC50=50 nM, >or=30-fold selective vs P2Y4 and P2Y6). All modifications at the uracil base of UTP led to a decrease in potency at the P2Y4 receptor. A beta,gamma-dichloromethylene modification in the triphosphate chain of 5-bromo-UTP was tolerated by all three receptor subtypes, thus opening up a new strategy to obtain ectonucleotide diphosphohydrolase- and phosphatase-resistant P2Y2, P2Y4, and P2Y6 receptor agonists.


