TolcaponeCOMT inhibitor CAS# 134308-13-7 |
- Entacapone sodium salt
Catalog No.:BCC4107
CAS No.:1047659-02-8
- Entacapone
Catalog No.:BCC2217
CAS No.:130929-57-6
Quality Control & MSDS
Number of papers citing our products

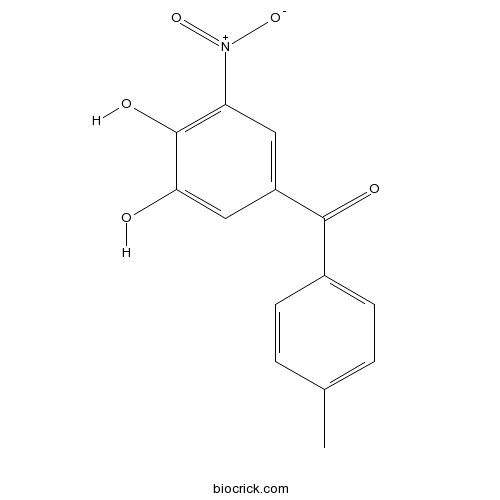
Chemical structure
3D structure
| Cas No. | 134308-13-7 | SDF | Download SDF |
| PubChem ID | 4659569 | Appearance | Powder |
| Formula | C14H11NO5 | M.Wt | 273.24 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | Tasmar, Ro 40-7592 | ||
| Solubility | Soluble to 100 mM in ethanol and to 100 mM in DMSO | ||
| Chemical Name | (3,4-dihydroxy-5-nitrophenyl)-(4-methylphenyl)methanone | ||
| SMILES | CC1=CC=C(C=C1)C(=O)C2=CC(=C(C(=C2)O)O)[N+](=O)[O-] | ||
| Standard InChIKey | MIQPIUSUKVNLNT-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C14H11NO5/c1-8-2-4-9(5-3-8)13(17)10-6-11(15(19)20)14(18)12(16)7-10/h2-7,16,18H,1H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | COMT inhibitor. Inhibits both brain and peripheral COMT. Also binds transthyretin (TTR) with high affinity (Kd1 and Kd2 values are 21 and 58 nM, respectively). Inhibits TTR aggregation in human plasma and prevents TTR-induced cytotoxicity in vitro. Stabilizes TTR in mice and humans in vivo. Orally bioavailable. |

Tolcapone Dilution Calculator

Tolcapone Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.6598 mL | 18.2989 mL | 36.5979 mL | 73.1957 mL | 91.4947 mL |
| 5 mM | 0.732 mL | 3.6598 mL | 7.3196 mL | 14.6391 mL | 18.2989 mL |
| 10 mM | 0.366 mL | 1.8299 mL | 3.6598 mL | 7.3196 mL | 9.1495 mL |
| 50 mM | 0.0732 mL | 0.366 mL | 0.732 mL | 1.4639 mL | 1.8299 mL |
| 100 mM | 0.0366 mL | 0.183 mL | 0.366 mL | 0.732 mL | 0.9149 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Tolcapone is a novel, reversible and orally-bioavailable small-molecule catechol-O-methyltransferase (COMT) inhibitor used for as an adjunct to levodopa therapy for the treatment of Parkinson’s disease (PD). The chemical structure of tolcapone contains a catechol structure with two electron withdrawing substituents of a tendency to easily deliver a proton resulting in an anion that is highly affinitive for COMT (the value of 50% inhibition concentration IC50 of 36 nM in rat liver) and displaces other catechols (such as catecholamines and levodopa) from the COMT catalytic center to prevent methylation. Study results have that the use of tolcapone reduces the dosage but enhances the therapeutic effects of levodopa to control PD symptoms.
Reference
Truong DD. Tolcapone: review of its pharmacology and use as adjunctive therapy in patients with Parkinson's disease. Clin Interv Aging. 2009;4:109-113
Jorga K, Fotteler B, Heizmann P, Gasser R. Metabolism and excretion of tolcapone, a novel inhibitor of catechol-O-methyltransferase. Br J Clin Pharmacol. 1999; 48(4):513-520.
- BIMU 8
Catalog No.:BCC7928
CAS No.:134296-40-5
- 3,5-Dibromo-4-[3-(dimethylamino)propoxy]cinnamic acid
Catalog No.:BCN1582
CAS No.:134276-56-5
- Daphnelantoxin B
Catalog No.:BCN3228
CAS No.:134273-12-4
- RKI-1447
Catalog No.:BCC1903
CAS No.:1342278-01-6
- Methylcobalamin
Catalog No.:BCC5188
CAS No.:13422-55-4
- Fmoc-Tyr(PO3Bzl2)-OH
Catalog No.:BCC3566
CAS No.:134150-51-9
- INCB8761(PF-4136309)
Catalog No.:BCC1649
CAS No.:1341224-83-6
- TP-0903
Catalog No.:BCC6462
CAS No.:1341200-45-0
- Ponasterone A
Catalog No.:BCN6184
CAS No.:13408-56-5
- (S)-4-Carboxyphenylglycine
Catalog No.:BCC6603
CAS No.:134052-73-6
- (R)-4-Carboxyphenylglycine
Catalog No.:BCC6602
CAS No.:134052-68-9
- (RS)-4-Carboxy-3-hydroxyphenylglycine
Catalog No.:BCC6598
CAS No.:134052-66-7
- alpha,beta-Methyleneadenosine 5'-triphosphate trisodium salt
Catalog No.:BCC7603
CAS No.:1343364-54-4
- 2-ThioUTP tetrasodium salt
Catalog No.:BCC7625
CAS No.:1343364-70-4
- Epoxymicheliolide
Catalog No.:BCN8275
CAS No.:1343403-10-0
- Ro 0437626
Catalog No.:BCC7276
CAS No.:134362-79-1
- Epoxomicin
Catalog No.:BCC1235
CAS No.:134381-21-8
- Seocalcitol
Catalog No.:BCC1944
CAS No.:134404-52-7
- Dehydroandrographolide
Catalog No.:BCN1260
CAS No.:134418-28-3
- CA 074
Catalog No.:BCC1141
CAS No.:134448-10-5
- Discodermide
Catalog No.:BCN1834
CAS No.:134458-00-7
- BW-B 70C
Catalog No.:BCC7013
CAS No.:134470-38-5
- Richenoic acid
Catalog No.:BCN6185
CAS No.:134476-74-7
- Trimethylvinylammonium(1+)
Catalog No.:BCN1820
CAS No.:13448-18-5
Brain catechol-O-methyltransferase (COMT) inhibition by tolcapone counteracts recognition memory deficits in normal and chronic phencyclidine-treated rats and in COMT-Val transgenic mice.[Pubmed:26919286]
Behav Pharmacol. 2016 Aug;27(5):415-21.
The critical involvement of dopamine in cognitive processes has been well established, suggesting that therapies targeting dopamine metabolism may alleviate cognitive dysfunction. Catechol-O-methyl transferase (COMT) is a catecholamine-degrading enzyme, the substrates of which include dopamine, epinephrine, and norepinephrine. The present work illustrates the potential therapeutic efficacy of COMT inhibition in alleviating cognitive impairment. A brain-penetrant COMT inhibitor, Tolcapone, was tested in normal and phencyclidine-treated rats and COMT-Val transgenic mice. In a novel object recognition procedure, Tolcapone counteracted a 24-h-dependent forgetting of a familiar object as well as phencyclidine-induced recognition deficits in the rats at doses ranging from 7.5 to 30 mg/kg. In contrast, entacapone, a COMT inhibitor that does not readily cross the blood-brain barrier, failed to show efficacy at doses up to 30 mg/kg. Tolcapone at a dose of 30 mg/kg also improved novel object recognition performance in transgenic mice, which showed clear recognition deficits. Complementing earlier studies, our results indicate that central inhibition of COMT positively impacts recognition memory processes and might constitute an appealing treatment for cognitive dysfunction related to neuropsychiatric disorders.
Comparison of the inhibitory effects of tolcapone and entacapone against human UDP-glucuronosyltransferases.[Pubmed:27089846]
Toxicol Appl Pharmacol. 2016 Jun 15;301:42-9.
Tolcapone and entacapone are two potent catechol-O-methyltransferase (COMT) inhibitors with a similar skeleton and displaying similar pharmacological activities. However, entacapone is a very safe drug used widely in the treatment of Parkinson's disease, while Tolcapone is only in limited use for Parkinson's patients and needs careful monitoring of hepatic functions due to hepatotoxicity. This study aims to investigate and compare the inhibitory effects of entacapone and Tolcapone on human UDP-glucosyltransferases (UGTs), as well as to evaluate the potential risks from the view of drug-drug interactions (DDI). The results demonstrated that both Tolcapone and entacapone exhibited inhibitory effects on UGT1A1, UGT1A7, UGT1A9 and UGT1A10. In contrast to entacapone, Tolcapone exhibited more potent inhibitory effects on UGT1A1, UGT1A7, and UGT1A10, while their inhibitory potentials against UGT1A9 were comparable. It is noteworthy that the inhibition constants (Ki) of Tolcapone and entacapone against bilirubin-O-glucuronidation in human liver microsomes (HLM) are determined as 0.68muM and 30.82muM, respectively, which means that the inhibition potency of Tolcapone on UGT1A1 mediated bilirubin-O-glucuronidation in HLM is much higher than that of entacapone. Furthermore, the potential risks of Tolcapone or entacapone via inhibition of human UGT1A1 were quantitatively predicted by the ratio of the areas under the plasma drug concentration-time curve (AUC). The results indicate that Tolcapone may result in significant increase in AUC of bilirubin or the drugs primarily metabolized by UGT1A1, while entacapone is unlikely to cause a significant DDI through inhibition of UGT1A1.
Repositioning tolcapone as a potent inhibitor of transthyretin amyloidogenesis and associated cellular toxicity.[Pubmed:26902880]
Nat Commun. 2016 Feb 23;7:10787.
Transthyretin (TTR) is a plasma homotetrameric protein implicated in fatal systemic amyloidoses. TTR tetramer dissociation precedes pathological TTR aggregation. Native state stabilizers are promising drugs to treat TTR amyloidoses. Here we repurpose Tolcapone, an FDA-approved molecule for Parkinson's disease, as a potent TTR aggregation inhibitor. Tolcapone binds specifically to TTR in human plasma, stabilizes the native tetramer in vivo in mice and humans and inhibits TTR cytotoxicity. Crystal structures of Tolcapone bound to wild-type TTR and to the V122I cardiomyopathy-associated variant show that it docks better into the TTR T4 pocket than tafamidis, so far the only drug on the market to treat TTR amyloidoses. These data indicate that Tolcapone, already in clinical trials for familial amyloid polyneuropathy, is a strong candidate for therapeutic intervention in these diseases, including those affecting the central nervous system, for which no small-molecule therapy exists.
Right inferior frontal cortex activity correlates with tolcapone responsivity in problem and pathological gamblers.[Pubmed:28066708]
Neuroimage Clin. 2016 Dec 20;13:339-348.
Failures of self-regulation in problem and pathological gambling (PPG) are thought to emerge from failures of top-down control, reflected neurophysiologically in a reduced capacity of prefrontal cortex to influence activity within subcortical structures. In patients with addictions, these impairments have been argued to alter evaluation of reward within dopaminergic neuromodulatory systems. Previously we demonstrated that augmenting dopamine tone in frontal cortex via use of Tolcapone, an inhibitor of the dopamine-degrading enzyme catechol-O-methyltransferase (COMT), reduced delay discounting, a measure of impulsivity, in healthy subjects. To evaluate this potentially translational approach to augmenting prefrontal inhibitory control, here we hypothesized that increasing cortical dopamine tone would reduce delay discounting in PPG subjects in proportion to its ability to augment top-down control. To causally test this hypothesis, we administered the COMT inhibitor Tolcapone in a randomized, double-blind, placebo-controlled, within-subject study of 17 PPG subjects who performed a delay discounting task while functional MRI images were obtained. In this subject population, we found that greater BOLD activity during the placebo condition within the right inferior frontal cortex (RIFC), a region thought to be important for inhibitory control, correlated with greater declines in impulsivity on Tolcapone versus placebo. Intriguingly, connectivity between RIFC and the right striatum, and not the level of activity within RIFC itself, increased on Tolcapone versus placebo. Together, these findings support the hypothesis that Tolcapone-mediated increases in top-down control may reduce impulsivity in PPG subjects, a finding with potential translational relevance for gambling disorders, and for behavioral addictions in general.
Different in vivo properties of three new inhibitors of catechol O-methyltransferase in the rat.[Pubmed:1628144]
Br J Pharmacol. 1992 Mar;105(3):569-74.
1. We compared three new catechol O-methyltransferase (COMT) inhibitors (OR-611, Ro 40-7592 and CGP 28014; 10 and 30 mg kg-1, i.p.) in male rats given levodopa (L-DOPA, 50 mg kg-1, i.p.) and carbidopa ((-)-L-alpha-methyl dopa, 50 mg kg-1, i.p.). In some studies pretreatment with pargyline (80 mg kg-1, i.p.) was used to block the function of monoamine oxidase (MAO). 2. Decreases of hypothalamic and striatal 3-O-methyl-dopa (3-OMD) levels were used as measures of the inhibition of peripheral COMT. The inhibition of brain COMT activity was estimated by decreases of hypothalamic and striatal homovanillic acid (HVA) and 3-methoxytyramine (3-MT; after pargyline) levels. 3. The three COMT inhibitors studied had different individual characteristics. OR-611 was primarily a peripherally acting COMT inhibitor, decreasing 3-OMD levels in the striatum (to 31-52%) and in the hypothalamus (to 16-27%) both in the control and pargyline-treated animals at 1 and 3 h. It did not have any effect on brain HVA and 3-MT. 3. Ro 40-7592 was a broad spectrum COMT inhibitor decreasing striatal and hypothalamic 3-OMD (always to less than 30%), HVA (to less than 50%) and 3-MT levels (to less than 23%) significantly both at 1 and 3 h. It was more potent than OR-611. 4. CGP 28014 functioned as a weak COMT inhibitor in the periphery inhibiting 3-OMD formation only at 3 h. In contrast, it was fairly potent in decreasing the brain HVA and 3-MT levels at 1 h (to 37-22% and 42-35% in the striatum, and to 57-33% and 64-35% in the hypothalamus, respectively) but not at 3 h. Since CGP 28014, unlike OR-611 and Ro 40-7592, did not generally increase the brain DOPA, dopamine or DOPAC levels, it was not a typical COMT inhibitor.


