Leupeptin, MicrobialInhibitor of serine and cysteine proteases CAS# 103476-89-7 |
- Calpain Inhibitor I, ALLN
Catalog No.:BCC1233
CAS No.:110044-82-1
- Acetyl-Calpastatin (184-210) (human)
Catalog No.:BCC2350
CAS No.:123714-50-1
- CA 074
Catalog No.:BCC1141
CAS No.:134448-10-5
- Gabexate mesylate
Catalog No.:BCC2096
CAS No.:56974-61-9
- Camostat Mesilate
Catalog No.:BCC4894
CAS No.:59721-29-8
- E-64-c
Catalog No.:BCC3588
CAS No.:76684-89-4
Quality Control & MSDS
Number of papers citing our products

Chemical structure

3D structure
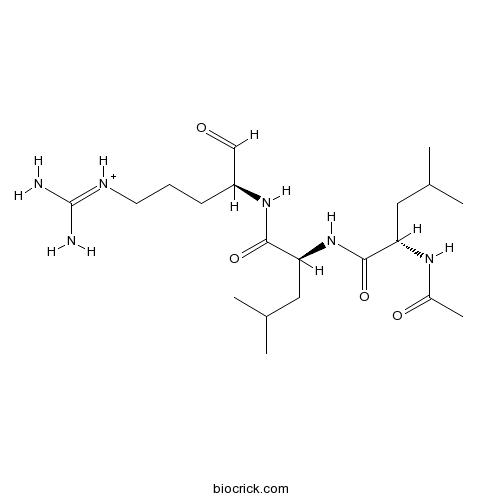
| Cas No. | 103476-89-7 | SDF | Download SDF |
| PubChem ID | 7098622 | Appearance | Powder |
| Formula | C20H39N6O4+ | M.Wt | 427.6 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | H2O : ≥ 19 mg/mL (39.95 mM) *"≥" means soluble, but saturation unknown. | ||
| Sequence | LLR (Modifications: Leu-1 = N-terminal Ac, Arg-3 = Arginal) | ||
| Chemical Name | [(4S)-4-[[(2S)-2-[[(2S)-2-acetamido-4-methylpentanoyl]amino]-4-methylpentanoyl]amino]-5-oxopentyl]-(diaminomethylidene)azanium | ||
| SMILES | CC(C)CC(C(=O)NC(CC(C)C)C(=O)NC(CCC[NH+]=C(N)N)C=O)NC(=O)C | ||
| Standard InChIKey | GDBQQVLCIARPGH-ULQDDVLXSA-O | ||
| Standard InChI | InChI=1S/C20H38N6O4/c1-12(2)9-16(24-14(5)28)19(30)26-17(10-13(3)4)18(29)25-15(11-27)7-6-8-23-20(21)22/h11-13,15-17H,6-10H2,1-5H3,(H,24,28)(H,25,29)(H,26,30)(H4,21,22,23)/p+1/t15-,16-,17-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Reversible inhibitor of trypsin-like and cysteine proteases such as calpain. Shown to inhibit activation-induced programmed cell death. |

Leupeptin, Microbial Dilution Calculator

Leupeptin, Microbial Molarity Calculator

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Leupeptin is a reversible inhibitor of protease with Ki values of 35 nM, 3.4 μM, 6 nM and 72 nM for bovine trypsin, human plasmin, bovine spleen cathepsin B and recombinant human calpain, respectively [1, 2].
As a protease inhibitor, leupeptin was originally isolated from the Streptomyces species. It exerted poor membrane permeability due to its polar C-terminal. For calpain, leupeptin showed moderate potent activities with IC50 values of 0.211 μM, 1.8 μM and 0.938 μM against the enzymes isolated from porcine erythrocyte, porcine kidney and human platelet, respectively. In cultured MRC-C cells, leupeptin suppressed the growth of human coronavirus strain 229E through inhibited the activity of trypsin. The mean IC50 value was 0.8 μM [2, 3].
References:
1. Mehdi S. Cell-penetrating inhibitors of calpain. Trends in biochemical sciences, 1991, 16: 150-153.
2. Krauser J A, Powers J C. 6.1 BIOLOGICAL ROLES. Proteinase and Peptidase Inhibition: Recent Potential Targets for Drug Development, 2003: 144.
3. Appleyard G, Tisdale M. Inhibition of the growth of human coronavirus 229E by leupeptin. Journal of general virology, 1985, 66(2): 363-366.
- Maprotiline HCl
Catalog No.:BCC4329
CAS No.:10347-81-6
- NMS-1286937
Catalog No.:BCC5358
CAS No.:1034616-18-6
- Xanthone V1a
Catalog No.:BCN7922
CAS No.:103450-96-0
- Z-Cyclopentyl-AP4
Catalog No.:BCC7616
CAS No.:103439-17-4
- CTAP
Catalog No.:BCC5776
CAS No.:103429-32-9
- CTOP
Catalog No.:BCC5780
CAS No.:103429-31-8
- Devazepide
Catalog No.:BCC7319
CAS No.:103420-77-5
- Disodium (R)-2-Hydroxyglutarate
Catalog No.:BCC6515
CAS No.:103404-90-6
- Octyl gallate
Catalog No.:BCN8432
CAS No.:1034-01-1
- 1-O-Methylnataloe-emodin
Catalog No.:BCN7036
CAS No.:103392-51-4
- HPGDS inhibitor 1
Catalog No.:BCC4065
CAS No.:1033836-12-2
- Telotristat
Catalog No.:BCC5128
CAS No.:1033805-28-5
- Diacetylpiptocarphol
Catalog No.:BCN4737
CAS No.:103476-99-9
- Fmoc-D-N- Me-Val-OH
Catalog No.:BCC3359
CAS No.:103478-58-6
- Fmoc-N-Me-Leu-OH
Catalog No.:BCC3345
CAS No.:103478-62-2
- Fmoc-D-N-Me-Leu-OH
Catalog No.:BCC3346
CAS No.:103478-63-3
- 9-Dehydroandrostenedione
Catalog No.:BCC8801
CAS No.:1035-69-4
- Phyllanthin
Catalog No.:BCN5848
CAS No.:10351-88-9
- AZ505
Catalog No.:BCC4264
CAS No.:1035227-43-0
- AZ505 ditrifluoroacetate
Catalog No.:BCC4265
CAS No.:1035227-44-1
- AZD4547
Catalog No.:BCC3711
CAS No.:1035270-39-3
- Apiosylskimmin
Catalog No.:BCN2455
CAS No.:103529-94-8
- Huperzine B
Catalog No.:BCN1059
CAS No.:103548-82-9
- 3,7-O-Diacetylpinobanksin
Catalog No.:BCN5849
CAS No.:103553-98-6
Purification and characterization of cathepsin L-like proteinase from goat brain.[Pubmed:22900325]
Indian J Biochem Biophys. 2003 Oct;40(5):315-23.
Cathepsin L-like proteinase was purified approximately 1708-fold with 40% activity yield to an apparent electrophoretic homogeneity from goat brain by homogenization, acid-autolysis at pH 4.2, 30-80% (NH4)2SO4 fractionation, Sephadex G-100 column chromatography and ion-exchange chromatography on CM-Sephadex C-50 at pH 5.0 and 5.6. The molecular weight of proteinase was found to be approximately 65,000 Da, by gel-filtration chromatography. The pH optima were 5.9 and 4.5 for the hydrolysis of Z-Phe-Arg-4mbetaNA (benzyloxycarbonyl-L-phenylalanine-L-arginine-4-methoxy-beta-naphthylamide) and azocasein, respectively. Of the synthetic chromogenic substrates tested, Z-Phe-Arg-4mbetaNA was hydrolyzed maximally by the enzyme (Km value for hydrolysis was 0.06 mM), followed by Z-Val-Lys-Lys-Arg-4mbetaNA, Z-Phe-Val-Arg-4mbetaNA, Z-Arg-Arg-4mbetaNA and Z-Ala-Arg-Arg-4mbetaNA. The proteinase was activated maximally by glutathione in conjunction with EDTA, followed by cysteine, dithioerythritol, thioglycolic acid, dithiothreitol and beta-mercaptoethanol. It was strongly inhibited by p-hydroxymercuribenzenesulphonic acid, iodoacetic acid, iodoacetamide and microbial peptide inhibitors, leupeptin and antipain. Leupeptin inhibited the enzyme competitively with Ki value 44 x 10(-9) M. The enzyme was strongly inhibited by 4 M urea. Metal ions, Hg(2+), Ca(2+), Cu(2+), Li(2+), K(+), Cd(2+), Ni(2+), Ba(2+), Mn(2+), Co(2+) and Sn(2+) also inhibited the activity of the enzyme. The enzyme was stable between pH 4.0-6.0 and up to 40 degrees C. The optimum temperature for the hydrolysis of Z-Phe-Arg-4mbetaNA was approximately 50-55 degrees C with an activation energy Ea of approximately 6.34 KCal mole(-1).
Characterization of proteases involved in the processing of Plasmodium falciparum serine repeat antigen (SERA).[Pubmed:11897123]
Mol Biochem Parasitol. 2002 Apr 9;120(2):177-86.
The Plasmodium falciparum serine repeat antigen (SERA), a malaria vaccine candidate, is processed into several fragments (P73, P47, P56, P50, and P18) at the late schizont stage prior to schizont rupture in the erythrocytic cycle of the parasite. We have established an in vitro cell-free system using a baculovirus-expressed recombinant SERA (bvSERA) that mimics the SERA processing that occurs in parasitized erythrocytes. SERA processing was mediated by parasite-derived trans-acting proteases, but not an autocatalytic event. The processing activities appeared at late schizont stage. The proteases are membrane associated, correlating with the secretion and accumulation of SERA within the parasitophorous vacuole membrane (PVM). The activity responsible for the primary processing step of SERA to P47 and P73 was inhibited by serine protease inhibitor DFP. In contrast, the activity responsible for the conversion of P56 into P50 was inhibited by each of the cysteine protease inhibitors E-64, leupeptin and iodoacetoamide. Moreover, addition of DFP, E-64 or leupeptin to the cultures of schizont-stage parasites blocked schizont rupture and release of merozoites from PVM. These results indicate that SERA processing correlates to schizont rupture and the processing is mediated by at least three distinct proteases.
Targeting Reactive Carbonyls for Identifying Natural Products and Their Biosynthetic Origins.[Pubmed:27797509]
J Am Chem Soc. 2016 Nov 23;138(46):15157-15166.
Natural products (NPs) serve important roles as drug candidates and as tools for chemical biology. However, traditional NP discovery, largely based on bioassay-guided approaches, is biased toward abundant compounds and rediscovery rates are high. Orthogonal methods to facilitate discovery of new NPs are thus needed, and herein we describe an isotope tag-based expansion of reactivity-based NP screening to address these shortcomings. Reactivity-based screening is a directed discovery approach in which a specific reactive handle on the NP is targeted by a chemoselective probe to enable its detection by mass spectrometry. In this study, we have developed an aminooxy-containing probe to guide the discovery of aldehyde- and ketone-containing NPs. To facilitate the detection of labeling events, the probe was dibrominated, imparting a unique isotopic signature to distinguish labeled metabolites from spectral noise. As a proof of concept, the probe was then utilized to screen a collection of bacterial extracts, leading to the identification of a new analogue of antipain, deimino-antipain. The bacterial producer of deimino-antipain was sequenced and the responsible biosynthetic gene cluster was identified by bioinformatic analysis and heterologous expression. These data reveal the previously undetermined genetic basis for a well-known family of aldehyde-containing, peptidic protease inhibitors, including antipain, chymostatin, leupeptin, elastatinal, and microbial alkaline protease inhibitor, which have been widely used for over 40 years.
Inhibitory effects of sword bean extract on alveolar bone resorption induced in rats by Porphyromonas gingivalis infection.[Pubmed:24494651]
J Periodontal Res. 2014 Dec;49(6):801-9.
BACKGROUND: The domesticated legume, Canavalia gladiata (commonly called the sword bean), is known to contain canavanine. The fruit is used in Chinese and Japanese herbal medicine for treating the discharge of pus, but its pharmacological mechanisms are still unclear. OBJECTIVES: This study examined the effect of sword bean extract (SBE) on (i) oral bacteria and human oral epithelial cells in vitro, and (ii) the initiation and progression of experimental Porphyromonas gingivalis-induced alveolar bone resorption in rats. MATERIAL AND METHODS: A high-performance liquid chromatography/ultraviolet method was applied to quantitate canavanine in SBE. By assessing oral bacterial growth, we estimated the minimum inhibitory concentration and minimum bactericidal concentration of SBE, canavanine, chlorhexidine gluconate (CHX) solution. The cytotoxicity of SBE, canavanine, CHX, leupeptin and cystatin for KB cells was determined using a trypan blue assay. The effects of SBE, canavanine, leupeptin and cystatin on Arg-gingipain (Rgp) and Lys-gingipain (Kgp) were evaluated by colorimetric assay using synthetic substrates. To examine its effects on P. gingivalis-associated periodontal tissue breakdown, SBE was orally administered to P. gingivalis-infected rats. RESULT: Sword bean extract contained 6.4% canavanine. SBE and canavanine inhibited the growth of P. gingivalis and Fusobacterium nucleatum. The cytotoxicity of SBE, canavanine and cystatin on KB cells was significantly lower than that of CHX. Inhibition of Rgp with SBE was comparable to that with leupeptin, a known Rgp inhibitor, and inhibition of Kgp with SBE was significantly higher than that with leupeptin at 500 mug/mL ( p < 0.05). P. gingivalis-induced alveolar bone resorption was significantly suppressed by administration of SBE, with bone levels remaining comparable to non-infected animals ( p < 0.05). CONCLUSION: The present study suggests that SBE might be effective against P. gingivalis-associated alveolar bone resorption.
A culture-based method for determining the production of secreted protease inhibitors.[Pubmed:24632514]
J Microbiol Methods. 2014 May;100:105-10.
We have developed a culture-based method for determining the production of secreted protease inhibitors. The assay utilizes standard proteolysis detection plates to support microbial growth followed by infiltrating the plate with a protease and subsequently detecting the remaining protein by trichloroacetic acid (TCA) precipitation, or by bromocreosol green (BCG) or Ponseau S (PS) staining. The presence of a protease inhibitor can be observed in the form of a protected zone of protein around the protease inhibitor-producing strain. Using the protease inhibitors alpha-2-macroglobulin, aprotinin, leupeptin, and bestatin and the primary and secondary forms of Photorhabdus luminescens in combination with the protease trypsin, we were able to demonstrate that the assay is specific for the cognate inhibitor of the protease and for bacteria secreting protease inhibitors. In addition, when casein-containing plates were used, the size of the diffusion zone was inversely correlated with the molecular weight of the inhibitor allowing a relative estimation of the protease inhibitor molecular weight. This assay is useful for detecting the presence of microbial secreted protease inhibitors and may reveal their production by microorganisms that were not previously recognized to produce them.
Inhibition of activation-induced programmed cell death and restoration of defective immune responses of HIV+ donors by cysteine protease inhibitors.[Pubmed:8021517]
J Immunol. 1994 Jul 15;153(2):862-72.
In vitro activation of PBLs from HIV+ individuals resulted in programmed cell death (PCD) within 2 days in 58 of 95 HIV+ blood donors, in contrast to only two of 30 control HIV- donors. CD4+ and CD8+ T cells from HIV+ donors died under these conditions, and these cells showed apoptotic nuclear morphology and DNA fragmentation. To test the hypothesis that this cell death shares a common biochemical pathway with that induced by TCR cross-linking in normal dividing T cells, inhibitors of the calcium-activated cysteine protease calpain were tested for their ability to block the activation-induced PCD of HIV+ donors. The E-64 (epoxysuccinyl) class of cysteine protease inhibitors gave 40% to 60% inhibition of HIV+ PCD responses, while the aldehyde inhibitors, leupeptin and calpain inhibitor II, gave 60% to 67% inhibition. The involvement of this calpain-dependent death pathway in HIV-induced functional T helper cell deficiency was tested by examining the effect of calpain inhibitors on the defective Ag- and mitogen-dependent proliferative responses of HIV+ donors. Twenty to fifty percent of such defective responses were significantly restored toward normal levels by calpain inhibitors, whereas control responses by normal donors were largely unaffected. These data suggest that a calpain-dependent PCD pathway contributes to HIV-associated immunodeficiency and suggest the use of calpain inhibitors as a possible route to therapy of HIV infection.
Cell-penetrating inhibitors of calpain.[Pubmed:1877091]
Trends Biochem Sci. 1991 Apr;16(4):150-3.
Inhibitors of the calcium-dependent cysteine protease calpain are described that are new analogs of the naturally-occurring compounds E-64 and leupeptin. These new derivatives, unlike the parent compounds, can inhibit calpain within cells. Their lack of charged groups probably accounts for this improved membrane permeability. These new inhibitors are proving useful in exploration of the role of calpain in many cellular processes, including platelet activation.


