CTOPPotent and selective μ antagonist CAS# 103429-31-8 |
- Stattic
Catalog No.:BCC1176
CAS No.:19983-44-9
- Fludarabine
Catalog No.:BCC2518
CAS No.:21679-14-1
- Cucurbitacin I
Catalog No.:BCC2439
CAS No.:2222-07-3
- WP1066
Catalog No.:BCC2194
CAS No.:857064-38-1
- SD 1008
Catalog No.:BCC2442
CAS No.:960201-81-4
Quality Control & MSDS
Number of papers citing our products

Chemical structure

3D structure
| Cas No. | 103429-31-8 | SDF | Download SDF |
| PubChem ID | 2884 | Appearance | Powder |
| Formula | C50H67N11O11S2 | M.Wt | 1062.28 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 1 mg/ml in water | ||
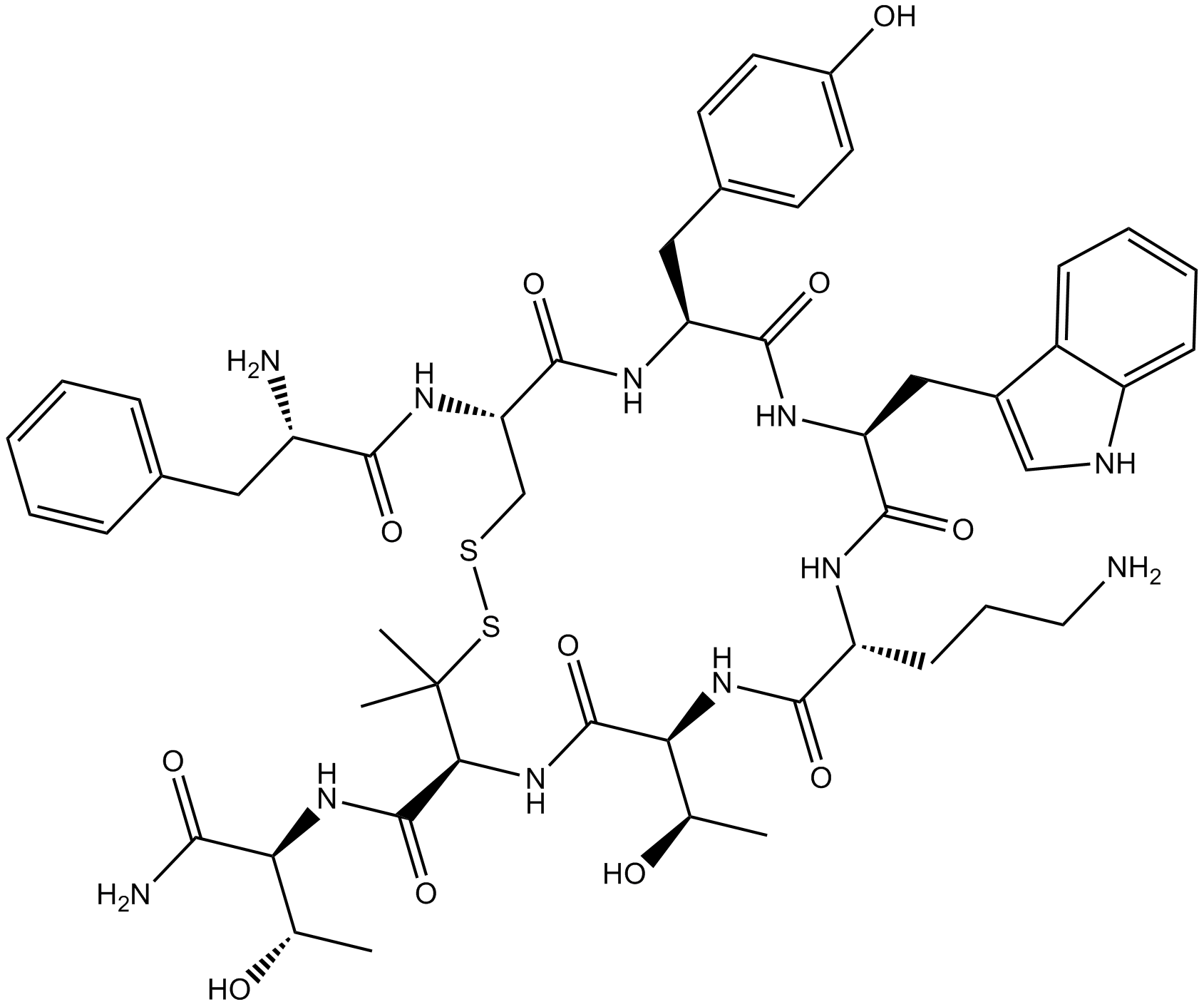
| Sequence | FCYWXTXT (Modifications: Phe-1 = D-Phe, Trp-4 = D-Trp, X-5 = Orn, X-7 = Pen, Disulfide bridge between 2 - 7, Thr-8 = C-terminal amide) | ||
| Chemical Name | N-(1-amino-3-hydroxy-1-oxobutan-2-yl)-19-[(2-amino-3-phenylpropanoyl)amino]-10-(3-aminopropyl)-7-(1-hydroxyethyl)-16-[(4-hydroxyphenyl)methyl]-13-(1H-indol-3-ylmethyl)-3,3-dimethyl-6,9,12,15,18-pentaoxo-1,2-dithia-5,8,11,14,17-pentazacycloicosane-4-carboxamide | ||
| SMILES | CC(C1C(=O)NC(C(SSCC(C(=O)NC(C(=O)NC(C(=O)NC(C(=O)N1)CCCN)CC2=CNC3=CC=CC=C32)CC4=CC=C(C=C4)O)NC(=O)C(CC5=CC=CC=C5)N)(C)C)C(=O)NC(C(C)O)C(=O)N)O | ||
| Standard InChIKey | PZWWYAHWHHNCHO-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C50H67N11O11S2/c1-26(62)39(42(53)65)59-49(72)41-50(3,4)74-73-25-38(58-43(66)33(52)21-28-11-6-5-7-12-28)47(70)56-36(22-29-16-18-31(64)19-17-29)45(68)57-37(23-30-24-54-34-14-9-8-13-32(30)34)46(69)55-35(15-10-20-51)44(67)60-40(27(2)63)48(71)61-41/h5-9,11-14,16-19,24,26-27,33,35-41,54,62-64H,10,15,20-23,25,51-52H2,1-4H3,(H2,53,65)(H,55,69)(H,56,70)(H,57,68)(H,58,66)(H,59,72)(H,60,67)(H,61,71) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Potent and selective μ opioid receptor antagonist (Ki values are 0.96 and >10,000 nM for μ and δ receptors respectively). Causes behavioral effects on central administration in vivo. Also increases K+ currents in rat locus ceruleus neurons in vitro via a μ receptor independent mechanism. |

CTOP Dilution Calculator

CTOP Molarity Calculator

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Devazepide
Catalog No.:BCC7319
CAS No.:103420-77-5
- Disodium (R)-2-Hydroxyglutarate
Catalog No.:BCC6515
CAS No.:103404-90-6
- Octyl gallate
Catalog No.:BCN8432
CAS No.:1034-01-1
- 1-O-Methylnataloe-emodin
Catalog No.:BCN7036
CAS No.:103392-51-4
- HPGDS inhibitor 1
Catalog No.:BCC4065
CAS No.:1033836-12-2
- Telotristat
Catalog No.:BCC5128
CAS No.:1033805-28-5
- Salidroside
Catalog No.:BCN5966
CAS No.:10338-51-9
- GNF-5837
Catalog No.:BCC3668
CAS No.:1033769-28-6
- Itol A
Catalog No.:BCN5847
CAS No.:1033747-78-2
- GNE-493
Catalog No.:BCC8048
CAS No.:1033735-94-2
- 1-Methyl-L-4,5-dihydroorotic acid
Catalog No.:BCC8472
CAS No.:103365-69-1
- L-364,373
Catalog No.:BCC7445
CAS No.:103342-82-1
- CTAP
Catalog No.:BCC5776
CAS No.:103429-32-9
- Z-Cyclopentyl-AP4
Catalog No.:BCC7616
CAS No.:103439-17-4
- Xanthone V1a
Catalog No.:BCN7922
CAS No.:103450-96-0
- NMS-1286937
Catalog No.:BCC5358
CAS No.:1034616-18-6
- Maprotiline HCl
Catalog No.:BCC4329
CAS No.:10347-81-6
- Leupeptin, Microbial
Catalog No.:BCC1217
CAS No.:103476-89-7
- Diacetylpiptocarphol
Catalog No.:BCN4737
CAS No.:103476-99-9
- Fmoc-D-N- Me-Val-OH
Catalog No.:BCC3359
CAS No.:103478-58-6
- Fmoc-N-Me-Leu-OH
Catalog No.:BCC3345
CAS No.:103478-62-2
- Fmoc-D-N-Me-Leu-OH
Catalog No.:BCC3346
CAS No.:103478-63-3
- 9-Dehydroandrostenedione
Catalog No.:BCC8801
CAS No.:1035-69-4
- Phyllanthin
Catalog No.:BCN5848
CAS No.:10351-88-9
Suppression of ethanol responding by centrally administered CTOP and naltrindole in AA and Wistar rats.[Pubmed:11198711]
Alcohol Clin Exp Res. 2001 Jan;25(1):25-33.
BACKGROUND: Both mu- and delta-opioid receptors have been implicated in the reinforcing actions of ethanol. However, selective opioid receptor antagonists have not altered ethanol intake in all rodent strains consistently, which suggests that genotype may modulate their suppressive effects. Therefore, we tested the effects of the selective mu-antagonist D-Pen-Cys-Tyr-D-Trp-Orn-Thr-Pen-Thr-NH2 (CTOP) and the selective delta-antagonist naltrindole in both high-drinking AA (Alko, Alcohol) and heterogeneous Wistar rats. METHODS: AA and Wistar rats were trained to respond for ethanol (10% w/v) in a two-lever operant condition by using a saccharin fading procedure. After stable baseline responding was established, rats were implanted stereotaxically either with a guide cannula above the lateral ventricle or with bilateral cannulas above the nucleus accumbens, basolateral amygdala, or ventral tegmental area. After postoperative recovery, AA and Wistar animals were tested after intracerebroventricular microinjections of either CTOP (0-3 microg) or naltrindole (0-30 microg) or subcutaneous injections of naloxone (0-1 g/kg), which was used as a reference antagonist. Effects of intracerebral microinjections of CTOP and naltrindole (both 0-500 ng) were tested only in Wistar rats. RESULTS: Subcutaneous naloxone and intracerebroventricular CTOP and naltrindole suppressed ethanol self-administration in a similar manner in AA and Wistar rats. Cumulative response patterns indicated that naloxone and naltrindole had no effect on the initiation of responding but suppressed it later during the session, whereas CTOP also affected initiation. In Wistar rats, naltrindole microinjections into both the nucleus accumbens and basolateral amygdala decreased ethanol responding, whereas CTOP was effective only in the amygdala. Injections of these antagonists into the ventral tegmental area had little effect on ethanol intake. CONCLUSIONS: The results confirm previous results which showed that both mu- and delta-opioid receptors are involved in the regulation of ethanol self-administration and indicate that genetic differences between AA and Wistar rats produced by selection do not modify the effects of opioid antagonists. The nucleus accumbens and the basolateral amygdala may be important central sites for the mediation of their suppressive effects.
Perceptions of adolescents in low resourced areas towards pregnancy and the Choice on Termination of Pregnancy (CTOP).[Pubmed:17515313]
Curationis. 2007 Mar;30(1):26-31.
Teenage pregnancy, unsafe abortion methods and the high incidence of HIV infections among young people are of great concern to the South African public. Due to the lack of accurate information and understanding, some adolescents are forced to succumb to early motherhood from unplanned pregnancies or opt for back-street abortion with at times fatal results. A qualitative exploratory study was conducted in 2003 to determine the adolescents' perceptions towards factors on the Choice on Termination of Pregnancy (CTOP) and the constraints in accessing TOP services. A purposive sampling technique that enabled experts such as health workers to identify suitable candidates for the investigation was employed. Twenty-four (24) adolescents residing in the predominantly rural area of Nkumpi-Lepelle in the Limpopo Province agreed to participate in the focus group interviews. The major findings indicated that most adolescents were uninformed about CTOP. This is attributed to the lack of coordination among health professionals and educators in the dissemination of information. The overwhelming majority of the respondents expressed discomfort at receiving termination of pregnancy services from the local public clinics and hospitals as they regarded such facilities as youth unfriendly. The adolescents also required provision of pre- and post-counselling services for adolescents who would like to terminate pregnancy. The following hypotheses were formulated for future in-depth studies: If adolescents continue to lack information about CTOP, they will not be able to utilize available services to terminate unplanned and unwanted pregnancies. If CTOP services remain inaccessible to the youth, the problem of backstreet abortion will not be eradicated.
Co-distribution of Fos- and mu opioid receptor immunoreactivity within the rat septopreoptic area and hypothalamus during acute glucose deprivation: effects of the mu receptor antagonist CTOP.[Pubmed:11406315]
Neurosci Lett. 2001 Jun 29;306(3):141-4.
Mu opioid receptors occur throughout the brain, but central sites where ligand neuromodulatory effects occur during glucopenia have not been identified. The present studies investigated whether septal, preoptic, and hypothalamic neurons that express immunoreactivity for this receptor are transcriptionally activated in response to the glucose antimetabolite, 2-deoxy-D-glucose (2DG), and if intracerebroventricular (icv) administration of the selective mu receptor antagonist, CTOP, modifies this functional response to glucose substrate imbalance. Neurons labeled for mu receptor-immunoreactivity (-ir) were observed in the lateral septal nucleus (LS), medial septum (MS), anterior division of the stria terminalis (BSTa), median preoptic nucleus (MEPO), medial preoptic nucleus (MPN), parastrial nucleus (PS), anterior hypothalamic periventricular nucleus (PVa), and lateral hypothalamic area (LPO). 2DG injection (400 mg/kg i.p.) resulted in co-labeling of mu receptor-positive neurons in the LS, MS, BSTa, MEPO, PVa, and LPO for nuclear Fos-ir. Icv delivery of CTOP decreased mean numbers of co-labeled neurons in the LS, MS, BSTa, and MEPO. These results provide evidence for transactivational effects of glucopenia on mu opioid receptor-expressing neurons within the septum, preoptic area, and hypothalamus, and suggest that the functional status of these receptors within discrete septopreoptic sites may be critical for maximal glucoprivic induction of the Fos stimulus-transcription cascade within local cells. These results thus support the view that the neural loci described above may serve as substrates for regulatory effects of mu opioid receptor ligands on central compensatory activities during acute glucose deprivation.
[Treatment outcome analysis of CTOP or CHOP regimen in newly diagnosed aggressive non-Hodgkin's lymphoma patients-results of a prospective, open, randomized, multicenter clinical trial].[Pubmed:21223712]
Zhonghua Xue Ye Xue Za Zhi. 2010 Oct;31(10):649-53.
OBJECTIVE: To compare the efficacy and toxicity of CTOP and CHOP regimen for newly diagnosed aggressive non-Hodgkin's lymphoma (NHL) patients. METHOD: From Oct 2006 to Jun 2009, 196 patients enrolled into this clinical trial from 72 centers in China were randomized into CTOP or CHOP group. RESULTS: Of 154 patients evaluated, 105 assigned in CTOP group and 49 in CHOP. Complete remission (CR) rate was 73.3%, and response rate (RR) was 87.6% in CTOP group and CR rate 71.4%, RR 86.2% in CHOP group, respectively (both P > 0.05). For B cell lymphomas, there was no difference in outcome between the two groups, but for T cell lymphomas, CR was 71.1% in CHOP, being significantly higher than that of 58.8% in CHOP group. There was no difference in hematological toxicity, GI reaction, liver and kidney function abnormality, but the occurrence of grade 3-4 alopecia in CTOP group (12.4%) was significantly lower than that in CHOP group (40.8%). The progress-free survival and overall survival (PFS and OS) at 1-, 2-, 3-year in CTOP group were 79%, 64.8%, 51.4% and 82.9%, 70.5%, 58.1%respectively; while in CTOP group were 77.6%, 61.2%, 49% and 81.6%, 67.3%, 55.1% respectively. CONCLUSION: CTOP regimen has similar effectiveness to CHOP regimen in newly diagnosed aggressive NHL, but with less side effects, and better efficacy for T cell lymphomas.
The mu-opioid receptor antagonist D-Phe-Cys-Tyr-D-Trp-Orn-Thr-Pen-Thr-NH2 (CTOP) [but not D-Phe-Cys-Tyr-D-Trp-Arg-Thr-Pen-Thr-NH2 (CTAP)] produces a nonopioid receptor-mediated increase in K+ conductance of rat locus ceruleus neurons.[Pubmed:8794906]
Mol Pharmacol. 1996 Sep;50(3):650-5.
The somatostatin analogues D-Phe-Cys-Tyr-D-Trp-Orn-Thr-Pen-Thr-NH2 (CTOP) and D-Phe-Cys-Tyr-D-Trp-Arg-Thr-Pen-Thr-NH2 (CTAP) have been used widely as selective antagonists of mu-opioid receptors. Actions of CTOP and CTAP on the membrane properties of rat locus ceruleus neurons were studied using intracellular recordings of membrane currents in superfused brain slices. CTOP increased a K+ conductance with an EC50 of 560 nM. The maximal conductance increase produced by CTOP (10 microM) was similar to that produced by high concentrations of the mu-opioid agonists D-Ala-Met-enkephalinglyol (1 microM) and Met-enkephalin (10 microM), as well as an alpha 2-adrenoceptor agonist (UK14304, 3 microM) and somatostatin (1 microM). The K+ current produced by CTOP was not antagonized by naloxone (1 microM), suggesting it was not mediated by mu-opioid receptors. The K+ currents induced by high concentrations of CTOP desensitized to 42% of the initial maximum after prolonged superfusion (t1/2 = 247 sec). In the presence of fully desensitized CTOP responses, somatostatin (1 microM) still produced near-maximal K+ currents; i.e., there was no cross-desensitization, which suggests that CTOP might act on a receptor distinct from somatostatin receptors. However, the converse did not apply; high concentrations of CTOP (30 microM) did not produce any additional current in the presence of desensitized somatostatin responses. No cross-desensitization was observed between CTOP (10-30 microM) and Met-enkephalin (30 microM) or nociceptin (3 microM) regardless of the order of drug application. Cyclo-(7-aminoheptanoyl-Phe-D-Trp-Lys-Thr[Bzl], antagonized both somatostatin-(KD = 10 microM) and CTOP-(KD = 8 microM) induced K+ currents with similar potency. Concentrations of CTOP (100 nM) that produced a small K+ current partially antagonized the actions of Met-enkephalin (10 microM) on mu-opioid receptors. In contrast to CTOP, CTAP produced no K+ current at concentrations of 300 nM and 1 microM and little current at 10 microM. CTAP potently antagonized K+ currents produced by the mu-opioid receptor agonist D-Ala-Met-enkephalin-glyol, with an equilibrium dissociation constant of 4 nM (Schild analysis). CTAP did not antagonize K+ currents produced by CTOP or somatostatin. These results demonstrate that CTOP is a potent and efficacious agonist at nonopioid receptors, whereas CTAP is a potent mu-opioid receptor antagonist with little nonopioid agonist activity in rat locus ceruleus neurons. The receptor activated by CTOP has yet to be fully resolved but seems to be similar to the somatostatin type 2 receptor or perhaps to a receptor closely related to somatostatin or opioid receptors.
Intra-VTA injections of the mu-opioid antagonist CTOP enhance locomotor activity.[Pubmed:7496796]
Brain Res. 1995 Aug 28;690(1):112-6.
In this paper we report on the effects of microinjections of the mu-opioid antagonist CTOP (D-Pen-Cys-Tyr-D-Trp-Orn-Thr-Pen-Thr-NH2) into the ventral tegmental area (VTA) on activity and ingestive behavior in the rat. Intra-VTA CTOP (0.015, 0.15, and 1.5 nmol per side) dose-dependently increased activity, whereas it had no effect on feeding and drinking behavior. These results are consistent with previous reports that intra-VTA injections of CTOP enhance extracellular dopamine levels in the nucleus accumbens. Furthermore, we propose a model of VTA mu-opioid mechanisms that might account for these surprising effects of intra-VTA CTOP.
[3H]-[H-D-Phe-Cys-Tyr-D-Trp-Orn-Thr-Pen-Thr-NH2] ([3H]CTOP), a potent and highly selective peptide for mu opioid receptors in rat brain.[Pubmed:2563293]
J Pharmacol Exp Ther. 1989 Jan;248(1):73-80.
The cyclic, conformationally restricted octapeptide [3H]-[H-D-Phe-Cys-Tyr-D-Trp-Orn-Thr-Pen-Thr-NH2] ([3H]CTOP) was synthesized and its binding to mu opioid receptors was characterized in rat brain membrane preparations. Association rates (k+1) of 1.25 x 10(8) M-1 min-1 and 2.49 x 10(8) M-1 min-1 at 25 and 37 degrees C, respectively, were obtained, whereas dissociation rates (k-1) at the same temperatures were 1.93 x 10(-2) min-1 and 1.03 x 10(-1) min-1 at 25 and 37 degrees C, respectively. Saturation isotherms of [3H]CTOP binding to rat brain membranes gave apparent Kd values of 0.16 and 0.41 nM at 25 and 37 degrees C, respectively. Maximal number of binding sites in rat brain membranes were found to be 94 and 81 fmol/mg of protein at 25 and 37 degrees C, respectively. [3H]CTOP binding over a concentration range of 0.1 to 10 nM was best fit by a one site model consistent with binding to a single site. The general effect of different metal ions and guanyl-5'-yl-imidodiphosphate on [3H]CTOP binding was to reduce its affinity. High concentrations (100 mM) of sodium also produced a reduction of the apparent mu receptor density. Utilizing the delta opioid receptor specific peptide [3H]-[D-Pen2,D-Pen5]enkephalin, CTOP appeared to be about 2000-fold more specific for mu vs. delta opioid receptor than naloxone. Specific [3H]CTOP binding was inhibited by a large number of opioid or opiate ligands.(ABSTRACT TRUNCATED AT 250 WORDS)
Central effects of the potent and highly selective mu opioid antagonist D-Phe-Cys-Tyr-D-Trp-Orn-Thr-Pen-Thr-NH2 (CTOP) in mice.[Pubmed:2901358]
Eur J Pharmacol. 1988 Jun 10;150(3):355-60.
The ability of the selective cyclic mu-opioid receptor antagonist, D-Phe-Cys-Tyr-D-Trp-Orn-Thr-Pen-Thr-NH2 (CTOP), to inhibit the acute and chronic effects of morphine in vivo was studied in mice. Intracerebroventricular (i.c.v.) administration of CTOP antagonized the analgesic effect of morphine in a dose-dependent manner, as measured by the heat-irradiant (tail-flick) method. CTOP was more effective than naloxone in inhibiting analgesia on a molar basis. CTOP also antagonized the acute morphine-induced hypermotility. CTOP caused withdrawal hypothermia and a loss of body weight in morphine-dependent animals. After the development of morphine-induced chronic dependence, CTOP administered i.c.v. caused a dose-dependent loss of body weight and hypothermia, and was about 10-400 times more potent than naloxone. CTOP administered alone to drugnaive mice did not cause antinociception, changes in body weight or body temperature.


