PD 334581MEK1 inhibitor CAS# 548756-68-9 |
- Imatinib Mesylate (STI571)
Catalog No.:BCC1115
CAS No.:220127-57-1
- Dasatinib (BMS-354825)
Catalog No.:BCC1281
CAS No.:302962-49-8
- Saracatinib (AZD0530)
Catalog No.:BCC1166
CAS No.:379231-04-6
- Bosutinib (SKI-606)
Catalog No.:BCC1167
CAS No.:380843-75-4
- DPH
Catalog No.:BCC1538
CAS No.:484049-04-9
Quality Control & MSDS
Number of papers citing our products

Chemical structure

3D structure
| Cas No. | 548756-68-9 | SDF | Download SDF |
| PubChem ID | 5287529 | Appearance | Powder |
| Formula | C20H19F3IN5O2 | M.Wt | 545.3 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 100 mM in DMSO and to 10 mM in ethanol | ||
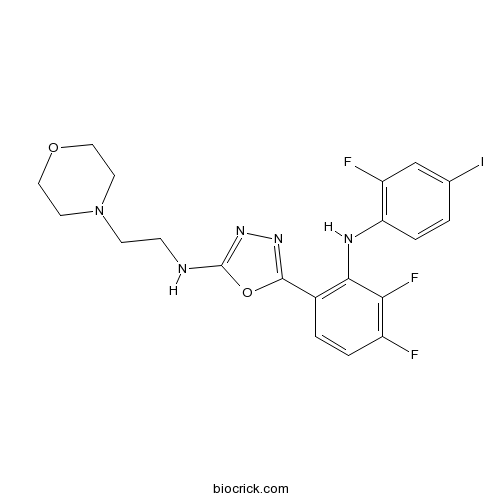
| Chemical Name | 5-[3,4-difluoro-2-(2-fluoro-4-iodoanilino)phenyl]-N-(2-morpholin-4-ylethyl)-1,3,4-oxadiazol-2-amine | ||
| SMILES | C1COCCN1CCNC2=NN=C(O2)C3=C(C(=C(C=C3)F)F)NC4=C(C=C(C=C4)I)F | ||
| Standard InChIKey | LZZYEMSEMRUPIM-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C20H19F3IN5O2/c21-14-3-2-13(18(17(14)23)26-16-4-1-12(24)11-15(16)22)19-27-28-20(31-19)25-5-6-29-7-9-30-10-8-29/h1-4,11,26H,5-10H2,(H,25,28) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Inhibitor of MEK1. Analog of PD 184352. |

PD 334581 Dilution Calculator

PD 334581 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.8339 mL | 9.1693 mL | 18.3385 mL | 36.6771 mL | 45.8463 mL |
| 5 mM | 0.3668 mL | 1.8339 mL | 3.6677 mL | 7.3354 mL | 9.1693 mL |
| 10 mM | 0.1834 mL | 0.9169 mL | 1.8339 mL | 3.6677 mL | 4.5846 mL |
| 50 mM | 0.0367 mL | 0.1834 mL | 0.3668 mL | 0.7335 mL | 0.9169 mL |
| 100 mM | 0.0183 mL | 0.0917 mL | 0.1834 mL | 0.3668 mL | 0.4585 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Sanshodiol
Catalog No.:BCN6577
CAS No.:54854-91-0
- Protogracillin(P)
Catalog No.:BCC8352
CAS No.:54848-30-5
- Roseoside
Catalog No.:BCN5728
CAS No.:54835-70-0
- Daphneolone
Catalog No.:BCN3230
CAS No.:54835-64-2
- Protoveratrine B
Catalog No.:BCN2435
CAS No.:124-97-0
- Trichodesmine
Catalog No.:BCN2145
CAS No.:548-90-3
- Gyrophoric acid
Catalog No.:BCN5731
CAS No.:548-89-0
- Galangin
Catalog No.:BCN5730
CAS No.:548-83-4
- Pinobanksin
Catalog No.:BCN5729
CAS No.:548-82-3
- Tectorigenin
Catalog No.:BCN1019
CAS No.:548-77-6
- Irigenin
Catalog No.:BCN3849
CAS No.:548-76-5
- Quercetagetin-7-O-glucoside
Catalog No.:BCN6480
CAS No.:548-75-4
- Arborinine
Catalog No.:BCN7438
CAS No.:5489-57-6
- Amitriptyline HCl
Catalog No.:BCC5033
CAS No.:549-18-8
- 8-Oxyberberine
Catalog No.:BCN3135
CAS No.:549-21-3
- Quercetin 3-O-beta-D-xylopyranoside
Catalog No.:BCN2851
CAS No.:549-32-6
- beta-Yohimbine
Catalog No.:BCN5733
CAS No.:549-84-8
- Phytolaccagenic acid
Catalog No.:BCN8090
CAS No.:54928-05-1
- Shikalkin
Catalog No.:BCC8359
CAS No.:54952-43-1
- 2-Oxopomolic acid
Catalog No.:BCN5732
CAS No.:54963-52-9
- Florilenalin
Catalog No.:BCN6422
CAS No.:54964-49-7
- Albendazole
Catalog No.:BCC3718
CAS No.:54965-21-8
- Tamoxifen Citrate
Catalog No.:BCC4382
CAS No.:54965-24-1
- Physalin D
Catalog No.:BCN7919
CAS No.:54980-22-2
Co-expression of AFAP1-AS1 and PD-1 predicts poor prognosis in nasopharyngeal carcinoma.[Pubmed:28380458]
Oncotarget. 2017 Jun 13;8(24):39001-39011.
Nasopharyngeal carcinoma (NPC) carries a high potential for metastasis and immune escape, with a great risk of relapse after primary treatment. Through analysis of whole genome expression profiling data in NPC samples, we found that the expression of a long non-coding RNA (lncRNA), actin filament-associated protein 1 antisense RNA 1 (AFAP1-AS1), is significantly correlated with the immune escape marker programmed death 1 (PD-1). We therefore assessed the expression of AFAP1-AS1 and PD-1 in a cohort of 96 paraffin-embedded NPC samples and confirmed that AFAP1-AS1 and PD-1 are co-expressed in infiltrating lymphocytes in NPC tissue. Moreover, patients with high expression of AFAP1-AS1 or PD-1 in infiltrating lymphocytes were more prone to distant metastasis, and NPC patients with positive expression of both AFAP1-AS1 and PD-1 had the poorest prognosis. This study suggests that AFAP1-AS1 and PD-1 may be potential therapeutic targets in NPC and that patients with co-expression of AFAP1-AS1 and PD-1 may be ideal candidates for future clinical trials of anti-PD-1 immune therapy.
Mechanistic insight into the regioselectivity of Pd(ii)-catalyzed C-H functionalization of N-methoxy cinnamamide.[Pubmed:28379232]
Dalton Trans. 2017 Apr 19;46(16):5288-5296.
Computational studies have been applied to gain insight into the mechanism of Pd(ii) catalyzed alpha-C-H functionalization of N-methoxy cinnamamide. The results show that the whole catalytic cycle proceeds via sequential six steps, including (i) catalyst Pd(t-BuNC)2 oxidation with O2, (ii) O-H deprotonation, (iii) t-BuNC migratory insertion to the Pd-C bond, (iv) acyl migration, (v) C-H activation and (vi) reductive elimination. The regioselectivity for different C-H activation sites depends on the coordination structures of alpha-C or beta-C to the palladium(ii) center. The coordination of alpha-C to the palladium(ii) center shows a regular planar quadrilateral structure, which is stable. However, the beta-C coordinating to the palladium(ii) center mainly exhibits a distorted quadrilateral structure, which is relatively unstable. Thus, the barrier of alpha-C-H activation is much lower than that of beta-C-H activation. The present results provide a deep understanding of the site-selectivity of C-H activation.
Three 1D cyanide-bridged M(Ni, Pd, Pt)-Mn(II) Coordination Polymer: Synthesis, Crystal Structure and Magnetic Properties.[Pubmed:28380238]
Acta Chim Slov. 2017 Mac;64(1):215-220.
Three tetracyanide-containing building blocks K2[M(CN)4] (M = Ni, Pd, Pt) and one semi-closed macrocycle seven-coordinated manganese(II) compound have been employed to assemble cyanide-bridged heterometallic complexes, resulting in three cyanide-bridged MII-MnII complexes: [Mn(L)][Ni(CN)4] . 2H2O (1) [Mn(L)][Pd(CN)4] (2) and [Mn(L)][Pt(CN)4] (3) (L = 2,6-bis[1-(2-(N-methylamino)ethylimino)ethyl]pyridine). Single-crystal X-ray diffraction analysis shows their similar one-dimensional structure consisting of the alternating [Mn(L)]2+ species and [M(CN)4]2- building blocks, generating a cyanide-bridged neutral polymeric chain. In all three isostructural complexes the coordination geometry of manganese ion is a slightly distorted pentagonal-bipyramidal with the two cyanide nitrogen atoms at the trans positions and N5 coordinating mode at the equatorial plane from ligand L. Investigation over magnetic properties of these complexes reveals very weak antiferromagnetic interaction between neighboring Mn(II) ions bridged by the long NC-M-CN unit. A best-fit to the magnetic susceptibility of complexes 1-3 leads to the magnetic coupling constant of J = -0.081, -0.103 and -0.14 cm-1, respectively.
Pd-Catalyzed Autotandem Reactions with N-Tosylhydrazones. Synthesis of Condensed Carbo- and Heterocycles by Formation of a C-C Single Bond and a C horizontal lineC Double Bond on the Same Carbon Atom.[Pubmed:28379703]
Org Lett. 2017 Apr 21;19(8):2034-2037.
A new Pd-catalyzed autotandem reaction is introduced that consists of the cross-coupling of a benzyl bromide with a N-tosylhydrazone followed by an intramolecular Heck reaction with an aryl bromide. During the process, a single and a double C-C bond are formed on the same carbon atom. Two different arrangements for the reactive functional groups are possible, rendering great flexibility to the transformation. The same strategy led to 9-methylene-9H-fluorenes, 9-methylene-9H-xanthenes, 9-methylene-9,10-dihydroacridines, and also dihydropyrroloisoquinoline and dihydroindoloisoquinoline derivatives.
Targeting cancer with small molecule kinase inhibitors.[Pubmed:19104514]
Nat Rev Cancer. 2009 Jan;9(1):28-39.
Deregulation of kinase activity has emerged as a major mechanism by which cancer cells evade normal physiological constraints on growth and survival. To date, 11 kinase inhibitors have received US Food and Drug Administration approval as cancer treatments, and there are considerable efforts to develop selective small molecule inhibitors for a host of other kinases that are implicated in cancer and other diseases. Herein we discuss the current challenges in the field, such as designing selective inhibitors and developing strategies to overcome resistance mutations. This Review provides a broad overview of some of the approaches currently used to discover and characterize new kinase inhibitors.
Structures of human MAP kinase kinase 1 (MEK1) and MEK2 describe novel noncompetitive kinase inhibition.[Pubmed:15543157]
Nat Struct Mol Biol. 2004 Dec;11(12):1192-7.
MEK1 and MEK2 are closely related, dual-specificity tyrosine/threonine protein kinases found in the Ras/Raf/MEK/ERK mitogen-activated protein kinase (MAPK) signaling pathway. Approximately 30% of all human cancers have a constitutively activated MAPK pathway, and constitutive activation of MEK1 results in cellular transformation. Here we present the X-ray structures of human MEK1 and MEK2, each determined as a ternary complex with MgATP and an inhibitor to a resolution of 2.4 A and 3.2 A, respectively. The structures reveal that MEK1 and MEK2 each have a unique inhibitor-binding pocket adjacent to the MgATP-binding site. The presence of the potent inhibitor induces several conformational changes in the unphosphorylated MEK1 and MEK2 enzymes that lock them into a closed but catalytically inactive species. Thus, the structures reported here reveal a novel, noncompetitive mechanism for protein kinase inhibition.


