Ro 31-8220pan-PKC inhibitor CAS# 125314-64-9 |
- Zoledronic Acid
Catalog No.:BCC1067
CAS No.:118072-93-8
- Enzastaurin (LY317615)
Catalog No.:BCC1100
CAS No.:170364-57-5
- Chelerythrine chloride
Catalog No.:BCN8322
CAS No.:3895-92-9
- Staurosporine
Catalog No.:BCC3612
CAS No.:62996-74-1
Quality Control & MSDS
Number of papers citing our products

Chemical structure

3D structure
| Cas No. | 125314-64-9 | SDF | Download SDF |
| PubChem ID | 5083 | Appearance | Powder |
| Formula | C25H23N5O2S | M.Wt | 457.55 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | Bisindolylmaleimide IX | ||
| Solubility | Soluble in DMSO | ||
| Chemical Name | 3-[3-[4-(1-methylindol-3-yl)-2,5-dioxopyrrol-3-yl]indol-1-yl]propyl carbamimidothioate | ||
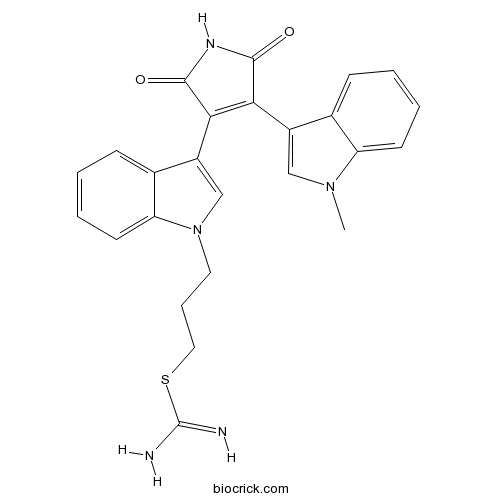
| SMILES | CN1C=C(C2=CC=CC=C21)C3=C(C(=O)NC3=O)C4=CN(C5=CC=CC=C54)CCCSC(=N)N | ||
| Standard InChIKey | DSXXEELGXBCYNQ-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C25H23N5O2S/c1-29-13-17(15-7-2-4-9-19(15)29)21-22(24(32)28-23(21)31)18-14-30(11-6-12-33-25(26)27)20-10-5-3-8-16(18)20/h2-5,7-10,13-14H,6,11-12H2,1H3,(H3,26,27)(H,28,31,32) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Ro 31-8220 is a inhibitor of pan-PKC with IC50 of 5 nM, 24 nM, 14 nM, 27 nM, and 24 nM for PKC-α, PKC-βI, PKC-βII, PKC-γ, and PKC-ε, respectively, | ||||||
| Targets | PKC-α | PKC-βII | PKC-βI | PKC-ε | PKC-γ | ||
| IC50 | 5 nM | 14 nM | 24 nM | 24 nM | 27 nM | ||

Ro 31-8220 Dilution Calculator

Ro 31-8220 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.1856 mL | 10.9278 mL | 21.8555 mL | 43.7111 mL | 54.6388 mL |
| 5 mM | 0.4371 mL | 2.1856 mL | 4.3711 mL | 8.7422 mL | 10.9278 mL |
| 10 mM | 0.2186 mL | 1.0928 mL | 2.1856 mL | 4.3711 mL | 5.4639 mL |
| 50 mM | 0.0437 mL | 0.2186 mL | 0.4371 mL | 0.8742 mL | 1.0928 mL |
| 100 mM | 0.0219 mL | 0.1093 mL | 0.2186 mL | 0.4371 mL | 0.5464 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Ro 31-8220 is a selective inhibitor of protein kinase C (PKC) with IC50 values of 5, 24, 14, 27, and 24 nM for PKC α, PKC βI, PKC βII, PKC γ and PKC ε, respectively [1].
PKC is a monomeric Ca2+- and phospholipid-dependent Ser/Thr protein kinases and plays an important role in growth factor-activated signaling and malignant transformation.
Ro 31-8220 is a selective inhibitor of PKC. Also, Ro 31-8220 increased growth factor-stimulated expression of c-Jun but inhibited MKP-1 and c-Fos expression. Ro-31-8220 strongly stimulated the stress-activated protein kinase JNK1 in a PKC-independent way [2]. In peripheral blood mononuclear cells, Ro 31-8220 inhibited the production of mitogen-induced interleukin (IL) -2 and IL-2-dependent T lymphoblast proliferation with IC50 values of 80 and 350 nM, respectively. Also, Ro 31-8220 (400 nM) inhibited IL-2Rα (CD25) expression by 27%, which suggested that Ro 31-8220 inhibited early and late T cell activation [3].
In rat adipocytes and L6 myotubes, RO 31-8220 activated c-Jun N-terminal kinase (JNK) and glycogen synthase (GS) and stimulated glucose incorporation into glycogen. While, RO 31-8220 inhibited the activity of extracellular response kinases 1 and 2 (ERK1/2) in a PKC-independent way [4].
References:
[1]. Wilkinson SE, Parker PJ, Nixon JS. Isoenzyme specificity of bisindolylmaleimides, selective inhibitors of protein kinase C. Biochem J, 1993, 294 ( Pt 2): 335-337.
[2]. Beltman J, McCormick F, Cook SJ. The selective protein kinase C inhibitor, Ro-31-8220, inhibits mitogen-activated protein kinase phosphatase-1 (MKP-1) expression, induces c-Jun expression, and activates Jun N-terminal kinase. J Biol Chem, 1996, 271(43): 27018-27024.
[3]. Geiselhart L, Conti DJ, Freed BM. RO 31-8220, a novel protein kinase C inhibitor, inhibits early and late T cell activation events. Transplantation, 1996, 61(11): 1637-1642.
[4]. Standaert ML, Bandyopadhyay G, Antwi EK, et al. RO 31-8220 activates c-Jun N-terminal kinase and glycogen synthase in rat adipocytes and L6 myotubes. Comparison to actions of insulin.
- Chlorantholide D
Catalog No.:BCN4825
CAS No.:1253106-58-9
- 21,24-Epoxycycloartane-3,25-diol
Catalog No.:BCN4718
CAS No.:125305-73-9
- Wallichinine
Catalog No.:BCN6602
CAS No.:125292-97-9
- 2,4,6-Trimethoxyphenol 1-O-beta-D-glucopyranoside
Catalog No.:BCN1593
CAS No.:125288-25-7
- Kihadanin A
Catalog No.:BCN3440
CAS No.:125276-62-2
- Spiranthesol
Catalog No.:BCN7915
CAS No.:125263-69-6
- Rubiprasin B
Catalog No.:BCN7137
CAS No.:125263-66-3
- Rubiprasin A
Catalog No.:BCN7138
CAS No.:125263-65-2
- (1beta,3beta,25S)-3-Hydroxyspirost-5-en-1-yl 2-O-(6-deoxy-alpha-L-mannopyranosyl)-beta-D-xylopyranoside
Catalog No.:BCN8164
CAS No.:125225-63-0
- Tubastatin A
Catalog No.:BCC2158
CAS No.:1252003-15-8
- 1,7-Bis(4-hydroxyphenyl)hept-6-en-3-one
Catalog No.:BCN6597
CAS No.:1251830-57-5
- Rosthornin B
Catalog No.:BCN6133
CAS No.:125181-21-7
- CD 437
Catalog No.:BCC7110
CAS No.:125316-60-1
- Vinorelbine Tartrate
Catalog No.:BCN2288
CAS No.:125317-39-7
- Pachysamine M
Catalog No.:BCN7309
CAS No.:1253202-75-3
- Periplogenin 3-[O-beta-glucopyranosyl-(1->4)-beta-sarmentopyranoside]
Catalog No.:BCN7861
CAS No.:1253421-94-1
- KS 176
Catalog No.:BCC7874
CAS No.:1253452-78-6
- N-Debenzoyl-N-(tert-butoxycarbonyl)taxol
Catalog No.:BCN1592
CAS No.:125354-16-7
- Antiquorin
Catalog No.:BCN7163
CAS No.:125356-08-3
- NMS-E973
Catalog No.:BCC5335
CAS No.:1253584-84-7
- AbK
Catalog No.:BCC8011
CAS No.:1253643-88-7
- 6-Acetonyl-N-methyl-dihydrodecarine
Catalog No.:BCN6134
CAS No.:1253740-09-8
- Cedrelone
Catalog No.:BCN6135
CAS No.:1254-85-9
- TCN 238
Catalog No.:BCC7901
CAS No.:125404-04-8
PKC inhibitors RO 31-8220 and Go 6983 enhance epinephrine-induced platelet aggregation in catecholamine hypo-responsive platelets by enhancing Akt phosphorylation.[Pubmed:21345315]
BMB Rep. 2011 Feb;44(2):140-5.
Impaired responsiveness of platelets to epinephrine (epi) and other catecholamines (CA) has been reported in approximately 20% of the healthy Korean and Japanese populations. In the present study, platelet aggregation induced by epi was potentiated by Ro 31-8220 (RO) or Go 6983 (Go). Phosphorylated Akt (p-Akt) was very low in epi-stimulated PRP from CA-hypo-responders (CA-HY), whereas it was detected in those from CA-good responders (CA-GR). RO and Go increased p-Akt, one of the major downstream effectors of phosphoinositol-3 kinase (PI3K), in epi-stimulated PRP from both groups. Wortmannin, a PI3K inhibitor, attenuated the RO or Go-induced potentiation of p-Akt in epi-stimulated PRP, suggesting positive effects for RO and Go on PI3K. TXA(2) formation was increased by the addition of either RO or Go in epi-stimulated platelets. The present data also suggest that impaired Akt phosphorylation may be responsible for epinephrine hypo-responsiveness of platelets.
Ro 31-6045, the inactive analogue of the protein kinase C inhibitor Ro 31-8220, blocks in vivo activation of p70(s6k)/p85(s6k): implications for the analysis of S6K signalling.[Pubmed:12023032]
FEBS Lett. 2002 May 22;519(1-3):135-40.
The mitogen-stimulated protein kinase p70(s6k)/p85(s6k) (S6K) plays an essential role in cell proliferation and growth, with inhibitors of the S6K signalling pathway showing promise as anti-tumour therapeutics. Here, we report that the bisindolylmaleimide derivative Ro 31-6045, previously reported to be inactive as a kinase inhibitor, inhibited S6K activity in vivo with an IC50=8 microM. Structure/function analysis using mutant forms of S6K indicates that Ro 31-6045 inhibition is independent of the upstream activator mTOR. Ro 31-6045 will prove useful in elucidating the complex activation mechanism of S6K and its independence from mTOR will allow confirmation of functional data obtained using the mTOR inhibitor rapamycin.
Exendin-4 as a stimulator of rat insulin I gene promoter activity via bZIP/CRE interactions sensitive to serine/threonine protein kinase inhibitor Ro 31-8220.[Pubmed:12021195]
Endocrinology. 2002 Jun;143(6):2303-13.
Signal transduction properties of exendin-4 (Ex-4) underlying its ability to stimulate rat insulin I gene promoter (RIP1) activity were assessed in the pancreatic beta-cell line INS-1. Ex-4 acted via glucagon-like peptide-1 receptors to stimulate RIP1 in a glucose-dependent manner, as measured in cells transfected with a -410-bp RIP1-luciferase construct (RIP1-Luc). The action of Ex-4 was independent of cAMP and PKA because it was not blocked by cotransfection with dominant-negative G alpha(s), was unaffected by pretreatment with the membrane-permeant cAMP antagonist 8-Br-Rp-cAMPS, and remained apparent after treatment with PKA inhibitors H-89 or KT 5720. Similarly, cotransfection with a dominant-negative isoform of the type-2 cAMP-regulated guanine nucleotide exchange factor (Epac2) failed to alter the response to Ex-4. Ro 31-8220, a serine/threonine protein kinase inhibitor that targets PKC as as well as the 90-kDa ribosomal S6 kinase (RSK) and mitogen- and stress-activated protein kinase (MSK) family of cAMP response element-binding protein (CREB) kinases, blocked the stimulatory action of Ex-4 at RIP1-Luc. However, selective inhibition of PKC using K-252c, prolonged exposure to phorbol 1,2-myristate-13-acetate, or cotransfection with dominant-negative atypical PKC-zeta, was without effect. A-CREB, a dominant-negative inhibitor of basic region-leucine zipper transcription factors (bZIPs) related in structure to CREB, inhibited the action of Ex-4 at RIP1-Luc, whereas A-ATF-2 was ineffective. Similarly, introduction of deletions at the RIP1 cAMP response element (CRE), or truncation of RIP1 to remove the CRE, nearly abolished the action of Ex-4. Inactivating mutations introduced at the A4/A3 elements, binding sites for the glucose-regulated homeodomain transcription factor PDX-1, did not diminish the response to Ex-4, although a marked reduction of basal promoter activity was observed. The glucose-dependent stimulation of RIP1-Luc by Ex-4 was reproduced using a synthetic reporter (RIP1-CRE-Luc) incorporating multimerized CREs of the RIP1 nonpalindromic sequence 5'-TGACGTCC-3'. It is concluded that the bZIP and CRE-mediated stimulation of RIP1 by Ex-4 explains, at least in part, how this insulinotropic hormone facilitates transcriptional activity of the rat insulin I gene.
Protein Kinase C-Independent Inhibition of Organic Cation Transporter 1 Activity by the Bisindolylmaleimide Ro 31-8220.[Pubmed:26657401]
PLoS One. 2015 Dec 10;10(12):e0144667.
Ro 31-8220 is a potent protein kinase C (PKC) inhibitor belonging to the chemical class of bisindolylmaleimides (BIMs). Various PKC-independent effects of Ro 31-8220 have however been demonstrated, including inhibition of the ATP-binding cassette drug transporter breast cancer resistance protein. In the present study, we reported that the BIM also blocks activity of the solute carrier organic cation transporter (OCT) 1, involved in uptake of marketed drugs in the liver, in a PKC-independent manner. Ro 31-8220, in contrast to other pan-PKC inhibitors such as staurosporine and chelerythrine, was thus shown to cis-inhibit uptake of the reference OCT1 substrate tetraethylammonium in OCT1-transfected HEK293 cells in a concentration-dependent manner (IC50 = 0.18 muM) and without altering membrane expression of OCT1. This blockage of OCT1 was also observed in human hepatic HepaRG cells that constitutionally express OCT1. It likely occurred through a mixed mechanism of inhibition. Ro 31-8220 additionally trans-inhibited TEA uptake in OCT1-transfected HEK293 cells, which likely discards a transport of Ro 31-8220 by OCT1. Besides Ro 31-8220, 7 additional BIMs, including the PKC inhibitor LY 333531, inhibited OCT1 activity, whereas 4 other BIMs were without effect. In silico analysis of structure-activity relationships next revealed that various molecular descriptors, especially 3D-WHIM descriptors related to total size, correspond to key physico-chemical parameters for inhibition of OCT1 activity by BIMs. In addition to activity of OCT1, Ro 31-8220 inhibited those of other organic cation transporters such as multidrug and toxin extrusion protein (MATE) 1 and MATE2-K, whereas, by contrast, it stimulated that of OCT2. Taken together, these data extend the nature of cellular off-targets of the BIM Ro 31-8220 to OCT1 and other organic cation transporters, which has likely to be kept in mind when using Ro 31-8220 and other BIMs as PKC inhibitors in experimental or clinical studies.


