(-)-SophoranoneCAS# 23057-55-8 |
Quality Control & MSDS
Number of papers citing our products

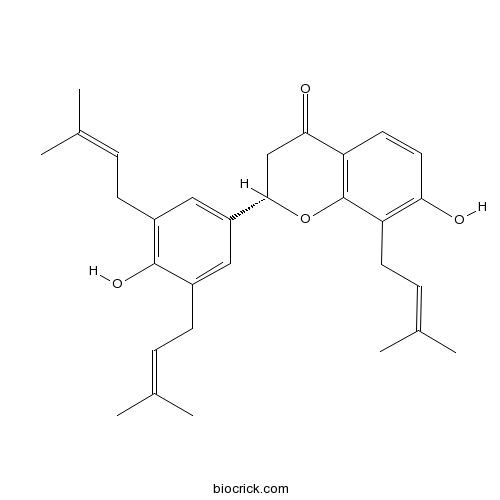
Chemical structure

3D structure
| Cas No. | 23057-55-8 | SDF | Download SDF |
| PubChem ID | 441767 | Appearance | White-yellow powder |
| Formula | C30H36O4 | M.Wt | 460.6 |
| Type of Compound | Flavonoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in diethyl ether and methan | ||
| Chemical Name | (2S)-7-hydroxy-2-[4-hydroxy-3,5-bis(3-methylbut-2-enyl)phenyl]-8-(3-methylbut-2-enyl)-2,3-dihydrochromen-4-one | ||
| SMILES | CC(=CCC1=CC(=CC(=C1O)CC=C(C)C)C2CC(=O)C3=C(O2)C(=C(C=C3)O)CC=C(C)C)C | ||
| Standard InChIKey | IORSRBKNYXPSDO-NDEPHWFRSA-N | ||
| Standard InChI | InChI=1S/C30H36O4/c1-18(2)7-10-21-15-23(16-22(29(21)33)11-8-19(3)4)28-17-27(32)25-13-14-26(31)24(30(25)34-28)12-9-20(5)6/h7-9,13-16,28,31,33H,10-12,17H2,1-6H3/t28-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | (-)-Sophoranone is a natural product from Sophora subprostrata. |
| Structure Identification | Journal of Separation Science, 2014, 37(22):3235-44.Simultaneous determination of trifolirhizin, (-)-maackiain, (-)-sophoranone, and 2-(2,4-dihydroxyphenyl)-5,6-methylenedioxybenzofuran from Sophora tonkinensis in rat plasma by liquid chromatography with tandem mass spectrometry and its application to a ph[Reference: WebLink]
|

(-)-Sophoranone Dilution Calculator

(-)-Sophoranone Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.1711 mL | 10.8554 mL | 21.7108 mL | 43.4216 mL | 54.277 mL |
| 5 mM | 0.4342 mL | 2.1711 mL | 4.3422 mL | 8.6843 mL | 10.8554 mL |
| 10 mM | 0.2171 mL | 1.0855 mL | 2.1711 mL | 4.3422 mL | 5.4277 mL |
| 50 mM | 0.0434 mL | 0.2171 mL | 0.4342 mL | 0.8684 mL | 1.0855 mL |
| 100 mM | 0.0217 mL | 0.1086 mL | 0.2171 mL | 0.4342 mL | 0.5428 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- L-AP4
Catalog No.:BCC6550
CAS No.:23052-81-5
- Dihydroconiferyl alcohol
Catalog No.:BCN7047
CAS No.:2305-13-7
- Lofepramine
Catalog No.:BCC7402
CAS No.:23047-25-8
- Ajugasterone C
Catalog No.:BCN2757
CAS No.:23044-80-6
- Z-Arg(NO2)-OH
Catalog No.:BCC3063
CAS No.:2304-98-5
- Z-Asn-OH
Catalog No.:BCC2794
CAS No.:2304-96-3
- Z-β-Ala-OH
Catalog No.:BCC3058
CAS No.:2304-94-1
- Terbutaline Sulfate
Catalog No.:BCC4320
CAS No.:23031-32-5
- Apelin-36 (rat, mouse)
Catalog No.:BCC5911
CAS No.:230299-95-3
- Physalin A
Catalog No.:BCN7920
CAS No.:23027-91-0
- Neoglycyrol
Catalog No.:BCN2907
CAS No.:23013-84-5
- MPTP hydrochloride
Catalog No.:BCC1778
CAS No.:23007-85-4
- Sinensetin
Catalog No.:BCN6356
CAS No.:2306-27-6
- Varenicline Hydrochloride
Catalog No.:BCC4156
CAS No.:230615-23-3
- Eurycomalactone
Catalog No.:BCN3108
CAS No.:23062-24-0
- PD 102807
Catalog No.:BCC7145
CAS No.:23062-91-1
- 4-Amino-N-methylphthalimide
Catalog No.:BCC8686
CAS No.:2307-00-8
- Xylazine HCl
Catalog No.:BCC4341
CAS No.:23076-35-9
- Sitosteryl palmitate
Catalog No.:BCN5078
CAS No.:2308-85-2
- 2-amino-3-(3-bromo-5-chloro-4-hydroxyphenyl)propanoic acid
Catalog No.:BCN8284
CAS No.:
- Corilagin
Catalog No.:BCN2322
CAS No.:23094-69-1
- Chebulagic acid
Catalog No.:BCN3262
CAS No.:23094-71-5
- Neuropeptide SF (mouse, rat)
Catalog No.:BCC6054
CAS No.:230960-31-3
- UK 356618
Catalog No.:BCC2378
CAS No.:230961-08-7
Pharmacokinetic properties of trifolirhizin, (-)-maackiain, (-)-sophoranone and 2-(2,4-dihydroxyphenyl)-5,6-methylenedioxybenzofuran after intravenous and oral administration of Sophora tonkinensis extract in rats.[Pubmed:26068519]
Xenobiotica. 2015;45(12):1092-104.
1. SKI3301, a standardized dried 50% ethanolic extracts of Sophora tonkinensis, contains four marker compounds (trifolirhizin, TF; (-)-maackiain, Maack; (-)-Sophoranone, SPN, and (2-(2,4-dihydroxyphenyl)-5,6-methylenedioxybenzofuran, ABF), is being developed as an herbal medicine for the treatment of asthma in Korea. This study investigates the pharmacokinetic properties of SKI3301 extract in rats. 2. The dose-proportional AUCs suggest linear pharmacokinetics of TF, Maack, SPN and ABF in the SKI3301 extract intravenous dose range of 5-20 mg/kg. After the oral administration of 200-1000 mg/kg of the extract, TF and Maack exhibited non-linearity due to the saturation of gastrointestinal absorption. However, linear pharmacokinetics of SPN and ABF were observed. 3. The absorptions of TF, Maack, SPN and ABF in the extract were increased relative to those of the respective pure forms due to the increased solubility and/or the decreased metabolism by other components in the SKI3301 extract. 4. No accumulation was observed after multiple dosing, and the steady-state pharmacokinetics of TF, Maack, SPN and ABF were not significantly different from those after a single oral administration of the extract. 5. The pharmacokinetics of TF, SPN and ABF were not significantly different between male and female rats after oral administration of the extract, but a significant gender difference in the pharmacokinetics of Maack in rats was observed. 6. Our findings may help to comprehensively elucidate the pharmacokinetic characteristics of TF, Maack, SPN and ABF and provide useful information for the clinical application of SKI3301 extract.
Simultaneous determination of trifolirhizin, (-)-maackiain, (-)-sophoranone, and 2-(2,4-dihydroxyphenyl)-5,6-methylenedioxybenzofuran from Sophora tonkinensis in rat plasma by liquid chromatography with tandem mass spectrometry and its application to a pharmacokinetic study.[Pubmed:25156071]
J Sep Sci. 2014 Nov;37(22):3235-44.
A new liquid chromatography with tandem mass spectrometry method was developed and validated for the simultaneous determination of trifolirhizin, (-)-maackiain, (-)-Sophoranone, and 2-(2,4-dihydroxyphenyl)-5,6-methylenedioxybenzofuran from Sophora tonkinensis in rat plasma using chlorpropamide as an internal standard. Plasma samples (50 muL) were prepared using a simple deproteinization procedure with 150 muL of acetonitrile containing 100 ng/mL of chlorpropamide. Chromatographic separation was carried out on an Acclaim RSLC120 C18 column (2.1 x 100 mm, 2.2 mum) using a gradient elution consisting of 7.5 mM ammonium acetate and acetonitrile containing 0.1% formic acid (0.4 mL/min flow rate, 7.0 min total run time). The detection and quantitation of all analytes were performed in selected reaction monitoring mode under both positive and negative electrospray ionization. This assay was linear over concentration ranges of 50-5000 ng/mL (trifolirhizin), 25-2500 ng/mL ((-)-maackiain), 5-250 ng/mL ((-)-Sophoranone), and 1-250 ng/mL 2-(2,4-dihydroxyphenyl)-5,6-methylenedioxybenzofuran) with a lower limit of quantification of 50, 25, 5, and 1 ng/mL for trifolirhizin, (-)-maackiain, (-)-Sophoranone, and 2-(2,4-dihydroxyphenyl)-5,6-methylenedioxybenzofuran, respectively. All the validation data, including the specificity, precision, accuracy, recovery, and stability conformed to the acceptance requirements. The results indicated that the developed method is sufficiently reliable for the pharmacokinetic study of the analytes following oral administration of Sophora tonkinensis extract in rats.


