Mitomycin CInhibits DNA synthesis,antibiotic and antitumor agent CAS# 50-07-7 |
- Gemcitabine HCl
Catalog No.:BCC1076
CAS No.:122111-03-9
- Proflavine Hemisulfate
Catalog No.:BCC4707
CAS No.:1811-28-5
- Lenalidomide (CC-5013)
Catalog No.:BCC2245
CAS No.:191732-72-6
- Carboxypeptidase G2 (CPG2) Inhibitor
Catalog No.:BCC1452
CAS No.:192203-60-4
- NSC 146109 hydrochloride
Catalog No.:BCC2410
CAS No.:59474-01-0
- Oxaliplatin
Catalog No.:BCC3932
CAS No.:61825-94-3
Quality Control & MSDS
Number of papers citing our products

Chemical structure
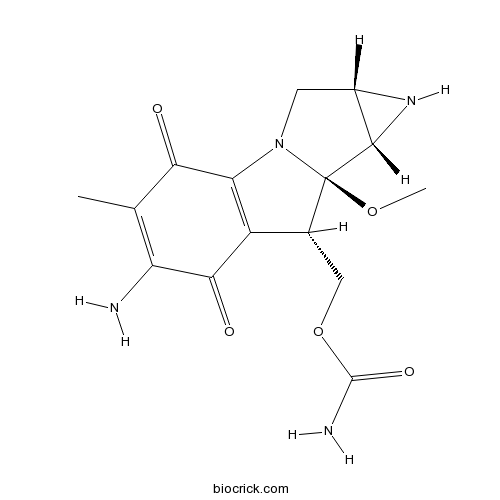
3D structure
| Cas No. | 50-07-7 | SDF | Download SDF |
| PubChem ID | 5746 | Appearance | Powder |
| Formula | C15H18N4O5 | M.Wt | 334.33 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | Ametycine | ||
| Solubility | DMSO : 50 mg/mL (114.71 mM; Need ultrasonic) | ||
| SMILES | CC1=C(C(=O)C2=C(C1=O)N3CC4C(C3(C2COC(=O)N)OC)N4)N | ||
| Standard InChIKey | NWIBSHFKIJFRCO-WUDYKRTCSA-N | ||
| Standard InChI | InChI=1S/C15H18N4O5/c1-5-9(16)12(21)8-6(4-24-14(17)22)15(23-2)13-7(18-13)3-19(15)10(8)11(5)20/h6-7,13,18H,3-4,16H2,1-2H3,(H2,17,22)/t6-,7+,13+,15-/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Antibiotic and antitumor agent. Covalently binds DNA forming intra- and interstrand crosslinks. Inhibits DNA synthesis. Also used for MEF/STO feeder layer preparation in stem cell culture. |

Mitomycin C Dilution Calculator

Mitomycin C Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.9911 mL | 14.9553 mL | 29.9106 mL | 59.8211 mL | 74.7764 mL |
| 5 mM | 0.5982 mL | 2.9911 mL | 5.9821 mL | 11.9642 mL | 14.9553 mL |
| 10 mM | 0.2991 mL | 1.4955 mL | 2.9911 mL | 5.9821 mL | 7.4776 mL |
| 50 mM | 0.0598 mL | 0.2991 mL | 0.5982 mL | 1.1964 mL | 1.4955 mL |
| 100 mM | 0.0299 mL | 0.1496 mL | 0.2991 mL | 0.5982 mL | 0.7478 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Mitomycin C, a kind of antibiotic isolated from Streptomyces caespitosus or Streptomyces lavendulae, inhibits DNA synthesis through covalent mitomycin C-DNA adduct with EC50 values of 0.14μM in PC3 cells. Therefore, it was served as a chemotherapeutic agent that has demonstrated its antitumor activity and has been used widely in treatment of various cancers. [1]
Mitomycin-C enhanced TRAIL (TNF-related apoptosis-inducing ligand)-induced apoptosis in p53 deficient colon cancer HCT116 cells. In the cell viability assay, pretreated with 5M with mitomycin C for 24h and then exposure to 25ng/ml TRAIL and 5M mitomycin C for 12h in HCT116 cells showed surprisingly decreased cell viability. In the crystal violet staining assay showed 5M mitomycin C combinated 25ng/ml TRAIL can substantially enhanced suppression effects of HCT116 cells. Pretreatment with 5M mitomycin C can enhanced TRAIL initiated processing of caspase-8, -9, -3 and cleavage of RARP (poly-ADP-ribose polymerase, substrate of caspase-3). The western blot assay showed in both HCT116 and HT29 cells, mitomycin C suppressed the expression anti apoptotic proteins Mcl-1, Bcl-2, Bcl-XL, and downregulated caspase-inhibitor c-IAP-1, XIAP, while upregulated expression of pro-apoptotic proteins Bax and Bim .[2]
The NMRI-Fox1nu nude mice was inoculated subcutaneously and then randomized to several groups: treated with electrochemotherapy and administrated 5mM mitomycin C or electrochemotherapy only or 5mM mitomycin C only. Results showed that tumor volume reduced in electrochemotherapy plus mitomycin C group, and mice survival rates were greater in electrochemotherapy plus mitomycin C group and mitomycin C group only, compared controls(p<0.001). The tumor response rate was 53% for mitomycin C alone.[3]
References:
[1] Danshiitsoodol N, de Pinho CA, Matoba Y, Kumagai T, Sugiyama M. The mitomycin C (MMC)-binding protein from MMC-producing microorganisms protects from the lethal effect of bleomycin: crystallographic analysis to elucidate the binding mode of the antibiotic to the protein. J Mol Biol (2006) 360 (2): 398–408
[2] Hairong Cheng, Bo Hong, Lanlan Zhou, Joshua E. Allen, Guihua Tai, Robin Humphreys,David T. Dicker, Yingqiu Y. Liu & Wafik S. El-Deiry. Mitomycin C potentiates TRAIL-induced apoptosis through p53-independent upregulation of death receptors. Cell Cycle (2012) 11(17):3312-3323
[3] Juan Luis Vásquez, Per Ibsen, Henriette Lindberg, Julie Gehl. In Vitro and In Vivo Experiments on Electrochemotherapy for Bladder Cancer. Journal of Urology (2014)
- Cortisone acetate
Catalog No.:BCC4771
CAS No.:50-04-4
- Dexamethasone (DHAP)
Catalog No.:BCC1184
CAS No.:50-02-2
- Guanidine HCl
Catalog No.:BCC4785
CAS No.:50-01-1
- Tioxolone
Catalog No.:BCC2316
CAS No.:4991-65-5
- 2,4-Pyridinedicarboxylic Acid
Catalog No.:BCC6483
CAS No.:499-80-9
- 5-Isopropyl-2-methylphenol
Catalog No.:BCN2633
CAS No.:499-75-2
- beta-Thujaplicin
Catalog No.:BCN3895
CAS No.:499-44-5
- IsoMaltose
Catalog No.:BCN8321
CAS No.:499-40-1
- Corydamine
Catalog No.:BCN3366
CAS No.:49870-84-0
- Erythroskyrin
Catalog No.:BCN1836
CAS No.:4987-27-3
- EX 527 (SEN0014196)
Catalog No.:BCC2223
CAS No.:49843-98-3
- Tobramycin Sulfate
Catalog No.:BCC5633
CAS No.:49842-07-1
- Ergocalciferol
Catalog No.:BCN2208
CAS No.:50-14-6
- Cyclophosphamide
Catalog No.:BCC1185
CAS No.:50-18-0
- Corticosterone
Catalog No.:BCN2203
CAS No.:50-22-6
- Hydrocortisone
Catalog No.:BCN2192
CAS No.:50-23-7
- Prednisolone
Catalog No.:BCC4830
CAS No.:50-24-8
- Estriol
Catalog No.:BCN2235
CAS No.:50-27-1
- beta-Estradiol
Catalog No.:BCN2194
CAS No.:50-28-2
- Phenylbutazone
Catalog No.:BCC4822
CAS No.:50-33-9
- Thalidomide
Catalog No.:BCC2248
CAS No.:50-35-1
- Cocaine
Catalog No.:BCN1901
CAS No.:50-36-2
- Clomiphene citrate
Catalog No.:BCC4480
CAS No.:50-41-9
- Mercaptopurine (6-MP)
Catalog No.:BCC1186
CAS No.:50-44-2
Initial Trabeculectomy With Mitomycin-C for Secondary Glaucoma-associated With Uveitis in Behcet Disease Patients.[Pubmed:28369000]
J Glaucoma. 2017 Jul;26(7):603-607.
PURPOSE: To examine clinical outcomes following an initial trabeculectomy with mitomycin-C for secondary glaucoma associated with uveitis in Behcet disease (BD) patients. DESIGN: Retrospective interventional case series. PATIENTS AND METHODS: Twenty-two eyes in 18 patients with uveitic glaucoma (UG) associated with Behcet disease who underwent an initial trabeculectomy with mitomycin-C between January 1996 and August 2014 were retrospectively reviewed. The main outcome measures were intraocular pressure (IOP) control, persistence of a filtering bleb, incidence of postoperative complications, and preopertaive and postoperative frequency of uveitic attacks. We analyzed persistence rates using Kaplan-Meier life tables based on 3 definitions of target IOP control (
XEN Glaucoma Implant with Mitomycin C 1-Year Follow-Up: Result and Complications.[Pubmed:28348884]
J Ophthalmol. 2017;2017:5457246.
Purpose. To evaluate gel microstent (XEN, Aquesys, Inc) for treatment of primary open angle glaucoma (POAG). Methods. In this prospective interventional study, 13 eyes with POAG underwent XEN implantation with subconjunctival mitomycin-C. Of those eyes, 3 were pseudophakic and 10 underwent simultaneous phacoemulsification and XEN. Patients had uncontrolled IOP, had intolerance to therapy, or had maximal therapy but undergoing cataract extraction. Follow-up visits included IOP, number of medications, vision, and complications and lasted for 1 year. Complete success was defined as IOP reduction >/=20% from preoperative baseline at 1 year without any glaucoma medications while partial success as IOP reduction of >/=20% at 1 year with medications. Results. IOP dropped from 16 +/- 4 mmHg pre-op to 9 +/- 5, 11 +/- 6, 12 +/- 5, 12 +/- 4, and 12 +/- 3 mmHg at 1 week, 1, 3, 6, and 12 months (p = 0.004, 0.026, 0.034, 0.01, and 0.01, Wilcoxon Signed Ranks) consecutively. BCVA (LogMAR) was 0.33 +/- 0.34 and improved to 0.13 +/- 0.11 at 1 year. Mean number of medications dropped from 1.9 +/- 1 preoperatively to 0.3 +/- 0.49 (p = 0.003) at 1 year. 42% of eyes achieved complete success and 66% qualified success. Complications included choroidal detachment in 2 eyes, and implant extrusion in 1 eye, and 2 eyes underwent trabeculectomy. Conclusion. XEN implant is an effective surgical treatment for POAG, with significant reduction in IOP and glaucoma medications at 1 year follow-up.
Preoperative subconjunctival combined injection of bevacizumab and mitomycin C before the surgical excision of primary pterygium: clinical and histological results.[Pubmed:28331283]
Clin Ophthalmol. 2017 Mar 10;11:493-501.
PURPOSE: The aim of this study was to detect the clinical and histological effects of preoperative subconjunctival injection of both bevacizumab and Mitomycin C (MMC) 1 month before the surgical excision of primary pterygium using a bare sclera technique. PATIENTS AND METHODS: A total of 20 patients with primary pterygium underwent subconjunctival combined injection of 0.1 mL of MMC (0.1 mg/mL) and 0.1 mL of bevacizumab (1.25 mg/0.1 mL) 1 month before bare sclera excision of the pterygium. The excised pterygium tissues were examined histologically and immunohistologically by CD31 staining, and the patients were followed up clinically for at least 2 years. The excised pterygia of two patients without preoperative injection were used for histological comparison. RESULTS: Clinically, there were no intraoperative or postoperative complications. No recurrence was noted during the follow-up period. Histologically, the previously injected pterygia showed a decreased number of epithelial cells and stromal fibroblasts. The latter were rounded or oval and swollen rather than spindle shaped, and some were degenerating or apoptotic. Collagen and elastic fibers were degenerated, distorted, and decreased in density, while blood capillaries were obliterated. There was a significant decrease in CD31-positive cells in previously injected pterygia. CONCLUSION: Preoperative subpterygium combined injection of bevacizumab and MMC is safe and effective in reducing the postoperative recurrence of primary pterygium. Histological and immunohistological changes in the form of decreased fibrovascular activity and degeneration of the extracellular matrix and nerve axons were noted.
Role of Mitomycin C in Preventing Capsular Contracture in Implant-Based Reconstructive Breast Surgery: A Randomized Controlled Trial.[Pubmed:28350652]
Plast Reconstr Surg. 2017 Apr;139(4):819-826.
BACKGROUND: Capsular contracture represents the most frequent complication after implant-based breast reconstruction. An experimental study on mice demonstrated that capsule formation around breast implants is considerably diminished after topical application of Mitomycin C. The authors conducted a randomized controlled clinical trial investigating the efficacy of Mitomycin C in reducing capsular contracture rates following implant-based breast reconstruction after mastectomy for breast cancer. METHODS: The authors randomized all women older than 18 years scheduled for the second stage of an implant-based breast reconstruction after mastectomy for breast cancer at the National Cancer Institute in Milan from October of 2005 to February of 2010 to receive or not receive the topical application of Mitomycin C during surgery. The authors assessed capsular contracture, major postoperative complications, and aesthetic outcome. RESULTS: The authors randomized 322 patients to receive Mitomycin C or not at the second stage of implant-based breast reconstruction. One hundred sixty-two patients were allocated to the Mitomycin C group and 160 patients were allocated to the control group. The relative risk of capsular contracture in the Mitomycin C group was 0.92 (95 percent CI, 0.60 to 1.41). Major complications leading to reintervention, oncologic outcomes, and aesthetic outcomes were comparable between the two groups. CONCLUSIONS: This is the first trial reporting data about the use of Mitomycin C in breast reconstructive surgery in a clinical setting. Mitomycin C seems not to significantly affect capsular contracture rate and severity following implant-based reconstructive breast surgery at the tested doses. CLINICAL QUESTION/LEVEL OF EVIDENCE: Therapeutic, I.
Modeling and correction of structural variations in patient-derived iPSCs using CRISPR/Cas9.[Pubmed:27711053]
Nat Protoc. 2016 Nov;11(11):2154-2169.
Genome engineering technology using engineered nucleases has been rapidly developing, enabling the efficient correction of simple mutations. However, the precise correction of structural variations (SVs) such as large inversions remains limited. Here we describe a detailed procedure for the modeling or correction of large chromosomal rearrangements and short nucleotide repeat expansions using engineered nucleases in human induced pluripotent stem cells (hiPSCs) from a healthy donor and patients with SVs. This protocol includes the delivery of engineered nucleases with no donor template to hiPSCs, and genotyping and derivation/characterization of gene-manipulated hiPSC clones. With engineered nucleases, genomic inversions, reversions, and deletions of short nucleotide expansions can be identified in 2 weeks, and desired clones can be generated in as little as 3-4 weeks. This protocol enables the correction of large inverted segments and short nucleotide repeat expansions in diseases such as hemophilia A, fragile X syndrome, Hunter syndrome, and Friedreich's ataxia.
Derivation of mouse embryonic stem cells.[Pubmed:17487198]
Nat Protoc. 2006;1(4):2082-7.
Here we describe a simple and efficient protocol for derivation of germline chimera-competent mouse embryonic stem cells (mESCs) from embryonic day 3.5 (E3.5) blastocysts. The protocol involves the use of early-passage mouse embryonic fibroblast feeders (MEF) and the alternation of fetal bovine serum- and serum replacement (SR)-containing media. As compared to other available protocols for mESCs derivation, our protocol differs in the combination of commercial availability of all reagents, technical simplicity and high efficiency. mESC lines are derived with approximately 50% efficiency (50 independent mESC lines derived from 96 blastocysts). We believe that this protocol could be a good starting point for (i) setting up the derivation of mESC lines in a laboratory and (ii) incorporating further steps to improve efficiency or adapt the protocol to other applications. The whole process (from blastocyst extraction to the freezing of mESC line) usually takes between 15 and 20 d.
Isolation and structure of an intrastrand cross-link adduct of mitomycin C and DNA.[Pubmed:1554696]
Biochemistry. 1992 Mar 31;31(12):3084-91.
A new covalent Mitomycin C-DNA adduct (4) was isolated from DNA exposed to reductively activated Mitomycin C (MC) in vitro. The MC-treated DNA was hydrolyzed enzymatically under certain conditions, and the new adduct was isolated from the hydrolysate by HPLC. Its structure was determined by ultraviolet and circular dichroism spectroscopy and chemical and enzymatic transformations conducted on microscale. In the structure, a single 2" beta, 7"-diaminomitosene residue is linked bifunctionally to two guanines in the dinucleoside phosphate d(GpG). The guanines are linked at their N2 atoms to the C1" and C10" positions of the mitosene, respectively. A key to the structure was a finding that removal of the mitosene from the adduct by hot piperidine yielded d(GpG); another was that the adduct was slowly converted to the known interstrand cross-link adduct 3 by snake venom diesterase and alkaline phosphatase. Adduct 4 represents an intrastrand cross-link in DNA formed by MC. Of the two possible strand-polarity isomers of 4, 4a in which the mitosene 1"-position is linked to the 3'-guanine of d(GpG) is designated as the proper structure, on the basis of the mechanism of the cross-linking reaction. The same adduct 4 was isolated from poly(dG).poly(dC), synthetic oligonucleotides containing the GpG sequence, and Micrococcus luteus and calf thymus DNAs. The relative yields of interstrand and intrastrand cross-links (3 and 4) were determined under first-order kinetic conditions; an average 3.6-fold preference for the formation of 3 over that of 4 was observed. An explanation for this preference is proposed.(ABSTRACT TRUNCATED AT 250 WORDS)
Isolation and structure of a covalent cross-link adduct between mitomycin C and DNA.[Pubmed:3103215]
Science. 1987 Mar 6;235(4793):1204-8.
A DNA cross-link adduct of the antitumor agent Mitomycin C (MC) to DNA has been isolated and characterized; the results provide direct proof for bifunctional alkylation of DNA by MC. Exposure of MC to Micrococcus luteus DNA under reductive conditions and subsequent nuclease digestion yielded adducts formed between MC and deoxyguanosine residues. In addition to the two known monoadducts, a bisadduct was obtained. Reductive MC activation with Na2S2O4 (sodium dithionite) leads to exclusive bifunctional alkylation. The structure of the bisadduct was determined by spectroscopic methods that included proton magnetic resonance, differential Fourier transform infrared spectroscopy, and circular dichroism. Formation of the same bisadduct in vivo was demonstrated upon injection of rats with MC. Computer-generated models of the bisadduct that was incorporated into the center of the duplex B-DNA decamer d(CGTACGTACG)2 indicated that the bisadduct fit snugly into the minor groove with minimal distortion of DNA structure. A mechanistic analysis of the factors that govern monofunctional and bifunctional adduct formation is presented.


