4'-O-MethylpyridoxineCAS# 1464-33-1 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 1464-33-1 | SDF | Download SDF |
| PubChem ID | 76581 | Appearance | Powder |
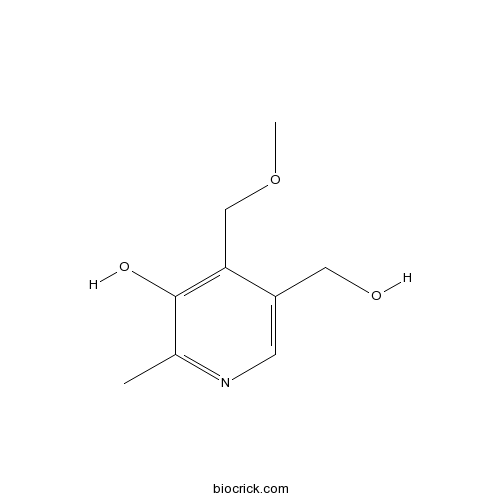
| Formula | C9H13NO3 | M.Wt | 183.2 |
| Type of Compound | Piperidines | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 5-(hydroxymethyl)-4-(methoxymethyl)-2-methylpyridin-3-ol | ||
| SMILES | CC1=NC=C(C(=C1O)COC)CO | ||
| Standard InChIKey | SVINQHQHARVZFF-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C9H13NO3/c1-6-9(12)8(5-13-2)7(4-11)3-10-6/h3,11-12H,4-5H2,1-2H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

4'-O-Methylpyridoxine Dilution Calculator

4'-O-Methylpyridoxine Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 5.4585 mL | 27.2926 mL | 54.5852 mL | 109.1703 mL | 136.4629 mL |
| 5 mM | 1.0917 mL | 5.4585 mL | 10.917 mL | 21.8341 mL | 27.2926 mL |
| 10 mM | 0.5459 mL | 2.7293 mL | 5.4585 mL | 10.917 mL | 13.6463 mL |
| 50 mM | 0.1092 mL | 0.5459 mL | 1.0917 mL | 2.1834 mL | 2.7293 mL |
| 100 mM | 0.0546 mL | 0.2729 mL | 0.5459 mL | 1.0917 mL | 1.3646 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Tortoside A
Catalog No.:BCN9131
CAS No.:190655-16-4
- Trilinolein
Catalog No.:BCN9130
CAS No.:537-40-6
- Methyl 2-(methylamino)benzoate
Catalog No.:BCN9129
CAS No.:85-91-6
- Silybin
Catalog No.:BCN9128
CAS No.:802918-57-6
- γ-Asarone
Catalog No.:BCN9127
CAS No.:5353-15-1
- Bacopaside N1
Catalog No.:BCN9126
CAS No.:871706-74-0
- 3'-O-Methylbatatasin III
Catalog No.:BCN9125
CAS No.:101330-69-2
- Arundinin
Catalog No.:BCN9124
CAS No.:148225-38-1
- Itaconic acid
Catalog No.:BCN9123
CAS No.:97-65-4
- Bacopaside IV
Catalog No.:BCN9122
CAS No.:155545-03-2
- Rapanone
Catalog No.:BCN9121
CAS No.:573-40-0
- Bletilloside A
Catalog No.:BCN9120
CAS No.:2292159-89-6
- Gingerglycolipid A
Catalog No.:BCN9133
CAS No.:145937-22-0
- Eupalinolide H
Catalog No.:BCN9134
CAS No.:1402067-83-7
- Regaloside F
Catalog No.:BCN9135
CAS No.:120601-65-2
- Regaloside C
Catalog No.:BCN9136
CAS No.:117591-85-2
- Cycloartenyl ferulate
Catalog No.:BCN9137
CAS No.:21238-33-5
- Triglochinic acid
Catalog No.:BCN9138
CAS No.:31795-12-7
- Methoxyeugenol 4-O-rhamnosyl(1→2)glucoside
Catalog No.:BCN9139
CAS No.:903519-86-8
- Sinapoyl sinapaldehyde
Catalog No.:BCN9140
CAS No.:
- 6′′-O-β-D-Apiofuranosylapterin
Catalog No.:BCN9141
CAS No.:2188162-94-7
- Epirengynic acid
Catalog No.:BCN9142
CAS No.:1310146-00-9
- 6-(3-Methyl-2-oxobutyroyl)-7-methoxycoumarin
Catalog No.:BCN9143
CAS No.:2188162-96-9
- 3,4-Dihydroxybenzoyllupeol
Catalog No.:BCN9144
CAS No.:2231323-99-0
An Adult Case of Generalized Convulsions Caused by the Ingestion of Ginkgo biloba Seeds with Alcohol.[Pubmed:32132337]
Intern Med. 2020 Jun 15;59(12):1555-1558.
A 64-year-old woman developed symptoms of vomiting and tonic-clonic convulsions 9.5 h after eating 50 roasted Ginkgo biloba seeds with 100 g of alcohol. The intravenous administration of pyridoxal phosphate effectively improved the symptoms. Blood samples were collected and stored over 35 h. The assessment of 4'-O-methylpyridoxine and vitamin B6 vitamers indicated high levels of both, but the pyridoxal phosphate levels were low during the acute stage. These results suggest that 4'-O-methylpyridoxine inhibits the transformation of vitamin B6 analogues to the active form, pyridoxal phosphate. In our case, alcohol may have extended the period until ginkgo intoxication appeared.
[Food Poisoning by Ginkgo Seeds through Vitamin B6 Depletion].[Pubmed:30606915]
Yakugaku Zasshi. 2019;139(1):1-6.
Overconsumption of Ginkgo biloba seeds induces food poisoning characterized by tonic-clonic convulsions and vomiting. The primary toxic component, 4'-O-methylpyridoxine (MPN), was purified from the seeds in 1985. This review includes the following aspects of ginkgo seed poisoning: 1) toxicity related to the content of MPN and MPN glucoside in G. biloba seeds; 2) the effect of MPN on vitamin B6 analogs, including an increase in pyridoxal and pyridoxic acid and decrease in pyridoxal-5'-phosphate plasma concentrations; 3) case reports of ginkgo seed poisoning in Asia, North America, and Europe, and their effective treatment via vitamin B6 administration. Considering the increase in the use of G. biloba seeds, it is essential to raise global awareness of their potential toxicity.
Concentrations of various forms of vitamin B6 in ginkgo seed poisoning.[Pubmed:30366747]
Brain Dev. 2019 Mar;41(3):292-295.
A 2-year-old girl required medical attention for a sudden onset of repetitive tonic-clonic convulsions after ingesting 20-30 ginkgo seeds. Concentrations of the major forms of circulating vitamin B6, pyridoxal-5'-phosphate (PLP), pyridoxal (PL), and 4-pyridoxic acid, as well as the known ginkgo seed toxin 4'-O-methylpyridoxine (MPN) were measured in the serum and cerebrospinal fluid (CSF). PLP is an active form of vitamin B6 and necessary for gamma-aminobutyric acid (GABA) production. High MPN concentrations were observed in both the serum and CSF. As the PLP to PL ratio was markedly decreased in serum and CSF examinations, we suspected the ratio to be important in GABA production. This case report provides novel information on the metabolism of vitamin B6 in humans as a result of ginkgo seed poisoning.
Interactions of pharmacokinetic profiles of Ginkgotoxin and Ginkgolic acids in rat plasma after oral administration.[Pubmed:30286439]
J Pharm Biomed Anal. 2019 Jan 30;163:88-94.
Ginkgolic acids (GAs) and Ginkgotoxin (4'-O-methylpyridoxine, MPN) are main toxic compounds in Ginkgo biloba seeds which are widely used in the treatment of coughing in China. To evaluate the pharmacokinetics of GAs, MPN and their metabolites in rat plasma, a highly sensitive method followed by ultra-high-pressure liquid chromatography coupled with linear ion trap-Orbitrap tandem mass spectrometry (UHPLC-LTQ-Orbitrap-MS) has been developed and validated. The proposed method is selective, precise and accurate enough of MPN and its metabolites (4-pyridoxic Acid, pyridoxal, and pyridoxine) for the pharmacokinetic study. After oral administration of MPN, the plasma concentrations of MPN and its metabolites were increased rapidly. Meanwhile, an investigation was carried out to compare the interactions of the pharmacokinetic profiles of MPN and GAs. Five GAs and main metabolites of GA (15:1) and GA (17:1) were also analyzed by using our previous method. After coadministration GAs with MPN, Tmax of MPN delayed and Cmax decreased. Meanwhile, Tmax of 4-pyridoxic Acid, pyridoxal, and pyridoxine were also showed a certain degree of delay. The concentrations of hydroxylation products of GA (15:1) and GA (17:1) increased at a slower rate and the area under the curves was significantly reduced. However, glucuronidation metabolites of GA (15:1) and GA (17:1) were increased faster than administered of GAs alone. The interactions of the pharmacokinetic profiles of GAs and MPN in rat plasma after oral administration were obviously observed.
Effects of roasting conditions on physicochemical properties and antioxidant activities in Ginkgo biloba seeds.[Pubmed:30263835]
Food Sci Biotechnol. 2018 Apr 7;27(4):1057-1066.
The roasting treatment has been used to extend the shelf life of food and improves its quality, and eliminate or reduce toxic. In this study, we investigate the changes in the physicochemical properties and antioxidant activities of Ginkgo biloba seeds (GBS) according to the roasting temperature and duration. As the roasting temperature and duration increased, the pH (from 7.32 to 6.31) and total cyanide content (from 1.49 to 0.70 microg/g) decreased, whereas the titratable acidity (from 0.39 to 0.84%) increased. The antioxidant activities increased rapidly at 210 degrees C according to the increase in the roasting temperature and duration. The 4'-O-methylpyridoxine (MPN) content in the 210 degrees C heat treatment group decreased by more than 70% compared to the MPN content in the control group. These results suggest that heat-treated GBS could be used in food materials and medicines for decreasing cyanide and MPN contents as well as for increasing antioxidant compound contents.
Effect of heat treatment on 4'-O-methylpyridoxine (MPN) content in Ginkgo biloba seed extract solution.[Pubmed:29572951]
J Sci Food Agric. 2018 Oct;98(13):5153-5156.
BACKGROUND: A vitamin B6 derivative, 4'-O-methylpyridoxine (MPN), is responsible for food poisoning by Ginkgo biloba seeds. In this study, we investigate the content of pyridoxine and MPN in MPN standard solution and G. biloba seed extract solution upon heat treatment in order to evaluate the reduction of toxic components in G. biloba seed by such treatment. RESULTS: Heat treatment was conducted at 90-150 degrees C for 0-60 min, and all samples were adjusted to the same concentration of 1 g L(-1) . The MPN content decreased to 994.92-563.69 mg kg(-1) for MPN standard solution and to 371.56-76.84 mg kg(-1) for G. biloba seed extract solution, and in both cases decreased even further with increasing heat treatment time. However, in all samples, except for the 90 degrees C heat treatment group, the pyridoxine content in MPN standard solution increased with increasing heat temperature and time; in addition, the extract solution showed a similar tendency. This may be the result of thermal degradation of MPN into pyridoxine. CONCLUSION: We can expect to improve the utilization of functional food materials by applying suitable heat treatment conditions and decreasing the MPN content of the G. biloba seed. (c) 2018 Society of Chemical Industry.
The Generally Useful Estimate of Solvent Systems (GUESS) method enables the rapid purification of methylpyridoxine regioisomers by countercurrent chromatography.[Pubmed:26680272]
J Chromatogr A. 2015 Dec 24;1426:248-51.
The TLC-based Generally Useful Estimate of Solvent Systems (GUESS) method was employed for countercurrent chromatography solvent system selection, in order to separate the three synthetic isomers: 3-O-methylpyridoxine, 4'-O-methylpyridoxine (ginkgotoxin), and 5'-O-methylpyridoxine. The Rf values of the three isomers indicated that ChMWat+2 (chloroform-methanol-water 10:5:5, v/v/v) was appropriate for the countercurrent separation. The isomer separation was highly selective and demonstrated that the TLC-based GUESS method can accelerate solvent system selection for countercurrent separation. Accordingly, the study re-emphasizes the practicality of TLC as a tool to facilitate the rapid development of new countercurrent and centrifugal partition chromatography methods for this solvent system. Purity and structure characterization of all samples was performed by quantitative (1)H NMR.
Decrease in pyridoxal-5'-phosphate concentration and increase in pyridoxal concentration in rat plasma by 4'-O-methylpyridoxine administration.[Pubmed:26092494]
Nutr Res. 2015 Jul;35(7):637-42.
Food poisoning from Ginkgo biloba seeds can cause epilepsy because of a decrease in gamma-aminobutyric acid (GABA) concentrations in the brain. We previously demonstrated that 4'-O-methylpyridoxine (MPN) is responsible for this observed toxicity of G biloba seeds; however, the mechanism for the decrease in GABA and plasma concentration profile of MPN has not been clarified. Our hypothesis is that MPN induces a decrease in vitamin B6 concentrations, resulting in a decrease in GABA concentration. This study aimed to characterize the plasma concentration profile of MPN and intrinsic vitamin B6 concentrations (pyridoxal [PL], PL-5'-phosphate [PLP], and 4-pyridoxic acid) using a rat model. Plasma concentrations of B6 vitamers after intravenous MPN administration (5 mg/kg) were determined using high-performance liquid chromatography with a fluorescence detector. The half-life of MPN (0.91 +/- 0.05 hours) was shorter in rats than the previously reported value in humans. We found a significant decrease in the plasma concentration of PLP, an active form of vitamin B6, after MPN administration. We also observed an increase in plasma PL and 4-pyridoxic acid concentrations; the increase in PL concentration may be caused by either metabolism of MPN to PL or by MPN-mediated inhibition of PL kinase. The present study is the first in vivo study showing relatively rapid elimination of MPN in rats and a decrease in plasma PLP concentration caused by MPN.
Quantification of a botanical negative marker without an identical standard: ginkgotoxin in Ginkgo biloba.[Pubmed:24432981]
J Nat Prod. 2014 Mar 28;77(3):611-7.
A new strategy for the analysis of natural products uses a combination of quantitative (1)H NMR (qHNMR) and adsorbent-free countercurrent separation (CS) methodology to establish a quantification method for ginkgotoxin (4'-O-methylpyridoxine) in Ginkgo biloba preparations. The target analyte was concentrated in a one-step CS process using the ChMWat +2 solvent system (CHCl3-MeOH-H2O, 10:5:5) and subsequently assayed by qHNMR. While commercial G. biloba seeds contained 59 mug of ginkgotoxin per seed, the compound was below the limit of detection (9 ppm) in a typical leaf extract. Due to the enrichment potential and loss-free operation of CS, the combination of CS and qHNMR is a generally suitable approach for threshold assays aimed at quantifying target compounds such as botanical negative markers at the low ppm level. As the proof of principle is demonstrated for relatively small CS capacities (20 mL, 1:40 loading) and modest NMR sensitivity (n = 16, 400 MHz, 5 mm RT probe), the approach can be adapted to quantification at the ppb level. The procedure enables the quantification of a botanical negative marker in the absence of identical reference material, which otherwise is a prerequisite for LC-based assays.


