8,9-Dehydrothymol isobutyrateCAS# 38146-79-1 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 38146-79-1 | SDF | Download SDF |
| PubChem ID | 11356419 | Appearance | Oil |
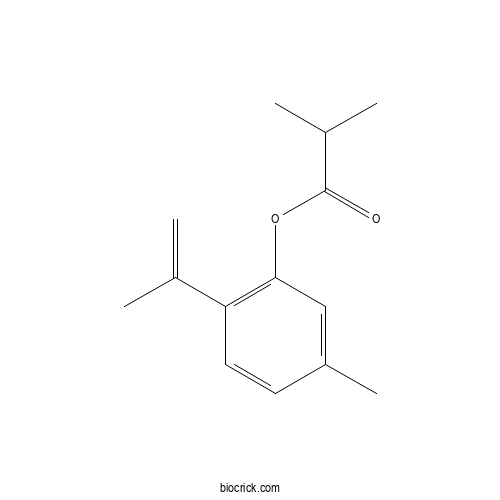
| Formula | C14H18O2 | M.Wt | 218.3 |
| Type of Compound | Other Terpenoid | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | (5-methyl-2-prop-1-en-2-ylphenyl) 2-methylpropanoate | ||
| SMILES | CC1=CC(=C(C=C1)C(=C)C)OC(=O)C(C)C | ||
| Standard InChIKey | BSAPRZRKFYAPEB-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C14H18O2/c1-9(2)12-7-6-11(5)8-13(12)16-14(15)10(3)4/h6-8,10H,1H2,2-5H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

8,9-Dehydrothymol isobutyrate Dilution Calculator

8,9-Dehydrothymol isobutyrate Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 4.5809 mL | 22.9043 mL | 45.8085 mL | 91.617 mL | 114.5213 mL |
| 5 mM | 0.9162 mL | 4.5809 mL | 9.1617 mL | 18.3234 mL | 22.9043 mL |
| 10 mM | 0.4581 mL | 2.2904 mL | 4.5809 mL | 9.1617 mL | 11.4521 mL |
| 50 mM | 0.0916 mL | 0.4581 mL | 0.9162 mL | 1.8323 mL | 2.2904 mL |
| 100 mM | 0.0458 mL | 0.229 mL | 0.4581 mL | 0.9162 mL | 1.1452 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- (+)-Perillaldehyde
Catalog No.:BCN0624
CAS No.:5503-12-8
- 1,7-Dimethoxyxanthone
Catalog No.:BCN0623
CAS No.:5042-06-8
- Swertianin 2-O-α-L-rhamnopyranosyl-(1→2)-β-D-xylopyranoside
Catalog No.:BCN0622
CAS No.:136832-00-3
- Bisisorhapontigenin C
Catalog No.:BCN0621
CAS No.:
- cis-Methylisoeugenol
Catalog No.:BCN0620
CAS No.:6380-24-1
- threo-1-(4-Hydroxyphenyl)propane-1,2,3-triol
Catalog No.:BCN0619
CAS No.:155748-73-5
- 6″-O-Acetylsaikosaponin b3
Catalog No.:BCN0618
CAS No.:104109-34-4
- 16-Oxolyclanitin-29-yl p-coumarate
Catalog No.:BCN0617
CAS No.:140701-70-8
- Thalmineline
Catalog No.:BCN0616
CAS No.:28328-00-9
- 2-Ethyl-3-methylmaleimide N-β-D-glucopyranoside
Catalog No.:BCN0615
CAS No.:182228-46-2
- Aristolactam IIIa N-β-glucoside
Catalog No.:BCN0614
CAS No.:80311-26-8
- 1,1'-[1,4-Phenylenebis(methylene)]bis[4,8,11-tris[(4-methylphenyl)sulfonyl]-1,4,8,11-tetraazacyclotetradecane
Catalog No.:BCN0613
CAS No.:110078-47-2
- 3-Hydroxy-p-menth-1-en-6-one
Catalog No.:BCN0626
CAS No.:61570-82-9
- 1,6,2',6'-O-Tetraacetyl-3-O-trans-p-coumaroylsucrose
Catalog No.:BCN0627
CAS No.:138195-49-0
- Ethyl 3,5-di-O-caffeoylquinate
Catalog No.:BCN0628
CAS No.:143051-74-5
- Ethyl 13-hydroxy-α-linolenate
Catalog No.:BCN0629
CAS No.:123435-84-7
- Campsiketalin
Catalog No.:BCN0630
CAS No.:93675-96-8
- Colelomycerone A
Catalog No.:BCN0631
CAS No.:1191896-73-7
- Annphenone
Catalog No.:BCN0632
CAS No.:61775-18-6
- (3S,5S)-[4]-Gingerdiol
Catalog No.:BCN0633
CAS No.:1448789-37-4
- Calophyllic acid
Catalog No.:BCN0634
CAS No.:36626-19-4
- Isocalophyllic acid
Catalog No.:BCN0635
CAS No.:157810-76-9
- Bisisorhapontigenin B
Catalog No.:BCN0636
CAS No.:
- 4-(4-Hydroxy-3-methoxyphenyl)butane-1,2-diol
Catalog No.:BCN0637
CAS No.:39115-22-5
Methodology for the Absolute Configuration Determination of Epoxythymols Using the Constituents of Piptothrix areolare.[Pubmed:33683122]
J Nat Prod. 2021 Mar 26;84(3):707-712.
Since epoxythymols occur in Nature either as scalemic mixtures or as pure enantiomers, the knowledge of their chiral composition and of the absolute configuration (AC) of the dominant enantiomer turns out to be mandatory. This task has already been faced using 1,1-bis-2-naphthol (BINOL), as a chiral solvating agent in accurate (1)H NMR quantifications to determine the enantiomeric ratio, and vibrational circular dichroism (VCD) to evidence the AC of the dominant enantiomer. We now explore the use of electronic circular dichroism (ECD) to determine the AC of an epoxythymol for which time-expensive DFT calculations would be required unless the AC of a related molecule is already known, from either VCD studies or single-crystal X-ray diffraction analysis, since one could correlate the ECD Cotton effect with the AC because in ECD only chromophores and their neighborhoods are evidenced. This method is now applied by using the epoxythymols from Piptothrix areolare. Known areolal (1) and 10-cinnamoyloxy-8,9-epoxythymol isobutyrate (2) were isolated from the roots, while known 7-acetoxy-10-cinnamoyloxy-8,9-epoxythymol isobutyrate (3) and 10-cinnamoyloxy-7-hydroxy-8,9-epoxythymol isobutyrate (4), as well as the new enantiopure 7-acetoxy-10-cinnamoyloxy-6-hydroxy-8,9-epoxythymol isobutyrate (5) and 10-cinnamoyloxy-8,9-epoxy-6-hydroxy-7-northymol isobutyrate (6), were obtained from the extract of the flowers. Chemical correlation of epoxythymols 1 and 3 was achieved. Compounds 1-4 were obtained as scalemic mixtures, and 5 and 6 as the pure (8S) enantiomers. In addition, the new 10-cinnamoyloxy-7-oxo-8,9-Dehydrothymol isobutyrate (7) was isolated from the roots. The structures of 5-7 followed from NMR and HRMS data, while enantiomeric compositions of 1-6 were determined by (1)H NMR-BINOL measurements. The AC determination for 2-6 was done by ECD using a sample of 1 to reference the ECD Cotton effect. In turn, the AC of 1 was determined by VCD and extensive DFT calculations. The ECD-BINOL methodology turned out to be some 500 times more sensitive than that combining VCD and (1)H NMR-BINOL.


