Alatusol DCAS# 1578237-42-9 |
Quality Control & MSDS
Package In Stock
Number of papers citing our products

| Cas No. | 1578237-42-9 | SDF | File under preparation. |
| PubChem ID | N/A | Appearance | Oil |
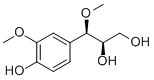
| Formula | C11H16O5 | M.Wt | 228.27 |
| Type of Compound | Phenylpropanoid | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Alatusol D Dilution Calculator

Alatusol D Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 4.3808 mL | 21.9039 mL | 43.8078 mL | 87.6155 mL | 109.5194 mL |
| 5 mM | 0.8762 mL | 4.3808 mL | 8.7616 mL | 17.5231 mL | 21.9039 mL |
| 10 mM | 0.4381 mL | 2.1904 mL | 4.3808 mL | 8.7616 mL | 10.9519 mL |
| 50 mM | 0.0876 mL | 0.4381 mL | 0.8762 mL | 1.7523 mL | 2.1904 mL |
| 100 mM | 0.0438 mL | 0.219 mL | 0.4381 mL | 0.8762 mL | 1.0952 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 2,4,6,1'-Tetra-O-acetyl-3',6'-di-O-feruloylsucrose
Catalog No.:BCX0566
CAS No.:173614-59-0
- Teucrenone
Catalog No.:BCX0565
CAS No.:152511-43-8
- Trichilinin D
Catalog No.:BCX0564
CAS No.:220698-24-8
- Isoconiferinoside
Catalog No.:BCX0563
CAS No.:152686-86-7
- 11-Hydroxyeremophil-1(10)-en-2-one
Catalog No.:BCX0562
CAS No.:20489-50-3
- Phaeocaulisin F
Catalog No.:BCX0561
CAS No.:1422201-43-1
- α-Tocotrienol
Catalog No.:BCX0560
CAS No.:58864-81-6
- 3,4'-Dihydroxypropiophenone 3-O-glucoside
Catalog No.:BCX0559
CAS No.:53170-92-6
- Sanggenol I
Catalog No.:BCX0558
CAS No.:202526-54-3
- 1-O-Caffeoyl-3,4,6-tri-O-galloyl-β-D-glucopyranose
Catalog No.:BCX0557
CAS No.:1219501-93-5
- 1,3-Di-O-caffeoyl-4-O-galloyl-β-D-glucopyranose
Catalog No.:BCX0556
CAS No.:359819-44-6
- Albonoursin
Catalog No.:BCX0555
CAS No.:1222-90-8
- α-Tocomonoenol
Catalog No.:BCX0568
CAS No.:97549-14-9
- 1-O-Caffeoyl-3-O-galloyl-4,6-O-hexahydroxydiphenoyl-β-D-glucopyranose
Catalog No.:BCX0569
CAS No.:359828-22-1
- Urodiolenone
Catalog No.:BCX0570
CAS No.:226547-04-2
- 3-Benzyl-6-benzylidenepiperazine-2,5-dione
Catalog No.:BCX0571
CAS No.:77027-30-6
- Piperafizine B
Catalog No.:BCX0572
CAS No.:74720-33-5
- Pyrroside A
Catalog No.:BCX0573
CAS No.:116271-36-4
- Ananasin A
Catalog No.:BCX0574
CAS No.:2242607-05-0
- Plicatinaphthol
Catalog No.:BCX0575
CAS No.:22127-07-7
- γ-Thujaplicatene
Catalog No.:BCX0576
CAS No.:29725-59-5
- Comososide
Catalog No.:BCX0577
CAS No.:1123534-26-8
- 3,4,6-Tri-O-galloyl-D-glucose
Catalog No.:BCX0578
CAS No.:99523-99-6
- Noueloside D
Catalog No.:BCX0579
CAS No.:2088336-76-7
Phenolic Constituents from Platycodon grandiflorum Root and Their Anti-Inflammatory Activity.[Pubmed:34361683]
Molecules. 2021 Jul 27;26(15):4530.
Six lignols (1-6), including two new compounds (+)-(7R,8R)-palmitoyl Alatusol D (1) and (+)-(7R,8R)-linoleyl Alatusol D (2), along with four phenolics (7-10), a neolignan (11), three alkyl aryl ether-type lignans (12-14), two furofuran-type lignans (15-16), three benzofuran-type lignans (17-19), a tetrahydrofuran-type lignan (20), and a dibenzylbutane-type lignan (21) were isolated from the ethyl acetate-soluble fraction of the methanol extract of Platycodon grandiflorum (Jacq.) A. DC. root. The chemical structures of the obtained compounds were elucidated via high-resolution mass spectrometry and nuclear magnetic resonance (NMR) spectroscopy analyses. The obtained spectroscopic data agreed well with literature. Among the isolated compounds, eighteen (1-7 and 11-21) were isolated from P. grandiflorum and the Campanulaceae family for the first time. This is the first report on lignol and lignan components of P. grandiflorum. The anti-inflammatory effects of the isolated compounds were examined in terms of their ability to inhibit the production of pro-inflammatory cytokines IL-6, IL-12 p40, and TNF-alpha in lipopolysaccharide-stimulated murine RAW264.7 macrophage cells. Nine compounds (4-6, 12, and 15-19) exhibited inhibitory effects on IL-12 p40 production, eleven compounds (1-6, 12, 15-17, and 19) exhibited inhibitory activity on IL-6 production, and eleven compounds (1-6 and 15-19) exhibited inhibitory effects against TNF-alpha. These results warrant further investigation into the potential anti-inflammatory activity and general benefits of the phenolic constituents of P. grandiflorum root.
Two pairs of phenylpropanoid enantiomers from the leaves of Eucommia ulmoides.[Pubmed:29996684]
J Asian Nat Prod Res. 2018 Nov;20(11):1045-1054.
Two pairs of phenylpropanoid enantiomers, (+)-(7S,8S)-Alatusol D (1a), (-)-(7R,8R)-Alatusol D (1b), (-)-(7S,8R)-Alatusol D (2a) and (+)-(7R,8S)-Alatusol D (2b) were isolated from the leaves of Eucommia ulmoides Oliver. Among them, 1a and 2b were firstly obtained by chiral enantiomeric resolution. Their structures were elucidated based on extensive spectroscopic analysis and the induced CD (ICD) spectrum caused by adding Mo(2)(AcO)(4) in DMSO. All compounds were tested on Hep G2 tumor cell lines. However, none of the compounds showed potential cytotoxic activity against Hep G2 in vitro.


