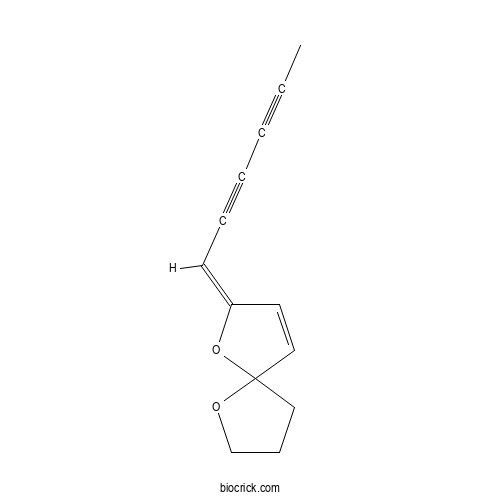
(E)-TonghaosuCAS# 50257-98-2 |
- (Z)-Tonghaosu
Catalog No.:BCX0104
CAS No.:4575-53-5
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 50257-98-2 | SDF | Download SDF |
| PubChem ID | 5352496 | Appearance | Oil |
| Formula | C13H12O2 | M.Wt | 200.2 |
| Type of Compound | Miscellaneous | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | (2E)-2-hexa-2,4-diynylidene-1,6-dioxaspiro[4.4]non-3-ene | ||
| SMILES | CC#CC#CC=C1C=CC2(O1)CCCO2 | ||
| Standard InChIKey | WTRXKCNFPMTAJV-KPKJPENVSA-N | ||
| Standard InChI | InChI=1S/C13H12O2/c1-2-3-4-5-7-12-8-10-13(15-12)9-6-11-14-13/h7-8,10H,6,9,11H2,1H3/b12-7+ | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

(E)-Tonghaosu Dilution Calculator

(E)-Tonghaosu Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 4.995 mL | 24.975 mL | 49.95 mL | 99.9001 mL | 124.8751 mL |
| 5 mM | 0.999 mL | 4.995 mL | 9.99 mL | 19.98 mL | 24.975 mL |
| 10 mM | 0.4995 mL | 2.4975 mL | 4.995 mL | 9.99 mL | 12.4875 mL |
| 50 mM | 0.0999 mL | 0.4995 mL | 0.999 mL | 1.998 mL | 2.4975 mL |
| 100 mM | 0.05 mL | 0.2498 mL | 0.4995 mL | 0.999 mL | 1.2488 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Quercetagetin 3-methyl ether 7-glucoside
Catalog No.:BCX0098
CAS No.:76060-29-2
- Raddeanoside R18
Catalog No.:BCX0097
CAS No.:781676-86-6
- 24-epi-24-O-acetyl-7,8-didehydrohydroshengmanol-3-O-beta-D-xylopyranoside
Catalog No.:BCX0096
CAS No.:228251-25-0
- Cimiricaside E
Catalog No.:BCX0095
CAS No.:2101977-10-8
- Patrinia saponin H3
Catalog No.:BCX0094
CAS No.:197013-75-5
- Rhamnocitrin-3-O-neohesperoside-4'-O-glucoside
Catalog No.:BCX0093
CAS No.:446276-95-5
- Norbergenin
Catalog No.:BCX0092
CAS No.:79595-97-4
- Tlatlancuayin
Catalog No.:BCX0091
CAS No.:3743-44-0
- 11-Oxomogroside IVa
Catalog No.:BCX0090
CAS No.:952481-54-8
- Ellagic acid 4-O-alpha-L-arabinofuranoside
Catalog No.:BCX0089
CAS No.:358617-39-7
- (E)-1-methoxy-2-O-(p-coumaroyl)-myo-inositol
Catalog No.:BCX0088
CAS No.:1391715-18-6
- Tectorigenin-7-O-beta-glucosyl-4'-O-beta-glucoside
Catalog No.:BCX0087
CAS No.:848128-32-5
- Raddeanoside R9
Catalog No.:BCX0100
CAS No.:124961-62-2
- 1-Methyl-6,8-dimethoxyquinoline-2 1H-one
Catalog No.:BCX0101
CAS No.:1210820-67-9
- alpha-[4-[(1E)-2-Carboxyethenyl]-2-hydroxyphenoxy]-beta,3,4-trihydroxybenzenepropanoic acid
Catalog No.:BCX0102
CAS No.:285136-03-0
- cis-2-Hydroxy 4-methoxycinnamic acid 2-glucoside
Catalog No.:BCX0103
CAS No.:150892-86-7
- (Z)-Tonghaosu
Catalog No.:BCX0104
CAS No.:4575-53-5
- N-trans-p-Coumaroyl-N'-trans-feruloyl-3-hydroxy-cadaverine
Catalog No.:BCX0105
CAS No.:2584997-91-9
- 4-(2-Hydroxyethyl)benzoic acid
Catalog No.:BCX0106
CAS No.:46112-46-3
- 2-Glucosyloxy-4-methoxycinnamic acid (Z-GMCA)
Catalog No.:BCX0107
CAS No.:31564-49-5
- 24-epi-7,8-Didehydrocimigenol 3-xyloside
Catalog No.:BCX0108
CAS No.:150972-77-3
- Isokaempferide 7-rutinoside
Catalog No.:BCX0109
CAS No.:18467-06-6
- 6-O-Galloylglucose
Catalog No.:BCX0110
CAS No.:13186-19-1
- Neobudofficide
Catalog No.:BCX0111
CAS No.:194602-91-0
Bioactive compounds from Matricaria chamomilla: structure identification, in vitro antiproliferative, antimigratory, antiangiogenic, and antiadenoviral activities.[Pubmed:34463438]
Z Naturforsch C J Biosci. 2021 Jun 22;77(3-4):85-94.
During our exploring the anticancer activity of some medicinal plants and their major metabolites, the aerial parts of the Egyptian Matricaria chamomilla (flowers and stems) were studied. GC-MS analysis of the organic soluble extracts of the flowers and stems fractions revealed the presence of 43 and 45 compounds, respectively. Individual chromatographic purification of the flowers and stems' extracts afforded three major compounds. Structures of these compounds were identified by 1D- and 2D-NMR and HRESI-MS spectroscopic data as bisabolol oxide A (1) and (E)-Tonghaosu (2) (as mixture of ratio 2:1) from the flowers extract, meanwhile apigenin-7-beta-d-glucoside (3) from the stems fraction. Biologically, the chamomile extracts announced significant antiproliferative activities exceeded in potency by approximately 1.5 fold in case of the stem, recording GI(50) 13.16 and 17.04 mug/mL against Caco-2 and MCF-7, respectively. Both fractions were approximately equipotent against the migration of the same cell type down to 10 mug/mL together, compounds 1, 2 but not 3, showed considerable growth inhibition of the same cells at GI(50) 13.36 and 11.83 mug/mL, respectively. Interestingly, they were able to suppress Caco-2 colon cancer cells migration at 5.8 mug/mL and potently inactivate the VEGFR2 angiogenic enzyme (1.5-fold relative to sorafenib. The obtained compounds and corresponding chamomile extracts were evaluated against Adeno-7 virus, revealing that both chamomiles' extracts (flowers and stems) and their corresponding obtained compounds (1-3) were potent in their depletion to the Adeno 7 infectivity titer, however, the flower extract and compounds 1-2 were more effective than those of the stem extract and its end-product (3).
Matricaria chamomilla (Chamomile) Ameliorates Muscle Atrophy in Mice by Targeting Protein Catalytic Pathways, Myogenesis, and Mitochondrial Dysfunction.[Pubmed:34247561]
Am J Chin Med. 2021;49(6):1493-1514.
Muscle atrophy, or loss of skeletal muscle, is caused by aging, malnutrition, immobility through injury, or diseases such as cancer. Chamomile (Matricaria chamomilla L.) contains various active components, including flavonoids, sesquiterpenes, polyacetylenes, and coumarins, and is used in various herbal medicines in the European Pharmacopoeia. In this study, we investigated the effects of ethanol extract of chamomile [Formula: see text](MC) on muscle wasting and its mechanism of action. Mice with dexamethasone (DEX)-induced muscle atrophy were orally administered MC (100, 200, and 300 mg/kg) for 4 weeks. Micro-computed tomography analysis showed that MC (200 and 300 mg/kg) significantly recovered DEX-induced loss of muscle volume, density, and weight and MC-treated DEX-induced mice also showed increased moving distance and grip strength. MC suppressed the mRNA level of muscle RING finger 1 (MuRF1) while increasing the expression of mitochondrial transcription factor A (TFAM), MyoD, and Myogenin-1. We found 25 peaks in MC samples through HPLC analysis and identified 6 peaks by comparison with a profile of standard compounds: chlorogenic acid (CGA), luteolin-7-O-glucoside (L7G), patulitrin, apigenin-7-O-glucoside (A7G), herniarin, and (E)-Tonghaosu. Of these components, the gene expression of MyoD was significantly augmented by patulitrin, herniarin, CGA, and L7G in C2C12 cells, while Myogenin-1 gene expression was increased by A7G, patulitrin, herniarin, CGA, and L7G. Moreover, TFAM gene expression and phosphorylation of AKT were increased by all six ingredients. Based on our results, we suggest MC for use as a supplement or remedy for muscle wasting, including cachexia and sarcopenia.
B-ring-homo-tonghaosu, isolated from Chrysanthemum morifolium capitulum, acts as a peroxisome proliferator-activated receptor-gamma agonist.[Pubmed:30790129]
J Nat Med. 2019 Jun;73(3):497-503.
The capitula of Chrysanthemum morifolium and C. indicum are used to prepare Chrysanthemi Flos in traditional Japanese Kampo medicine. In our previous study, we reported on the agonistic effect of methanol extract of C. indicum capitulum on peroxisome proliferator-activated receptor (PPAR)-gamma. We further isolated (E)-Tonghaosu from C. indicum capitulum as one of the active ingredients. In the present study, we aimed to evaluate the PPAR-gamma agonistic activity of a methanol extract of C. morifolium capitulum (MCM) in which (E)-Tonghaosu could not be detected. MCM exhibited PPAR-gamma agonistic activity in a concentration-dependent manner, and at a dose of 100 microg/ml, it showed similar activity to pioglitazone (30 microM), a standard PPAR-gamma agonist. Through activity-guided fractionation, we isolated two geometric isomers, (E)- (1) and (Z)-B-ring-homo-tonghaosu (2), as the active ingredients of MCM. Both compounds exerted concentration-dependent PPAR-gamma agonistic effects, and 1 had higher activity than 2. At 1.4 microM, 1 had similar activity to pioglitazone (30 microM), which was achieved by 2 at a concentration of 140 microM. Thus, 1 has the potential to become a lead compound for the drug discovery of PPAR-gamma agonists. We compared the activities and the contents of (E)-, (Z)-tonghaosu, 1, and 2 among 13 commercial samples of Chrysanthemi Flos, including those derived from both C. morifolium and C. indicum. Their PPAR-gamma agonistic activities were not related to the contents of these compounds. 1 and 2 were detected in the samples derived from both species but (E)- and (Z)-tonghaosu were not detected in the samples derived from C. morifolium; hence (E)- and (Z)-tonghaosu can serve as marker compounds to identify the capitula of C. indicum in Chrysanthemi Flos samples.


