Ethyl brevifolincarboxylateCAS# 107646-82-2 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 107646-82-2 | SDF | Download SDF |
| PubChem ID | 5487248 | Appearance | Yellow powder |
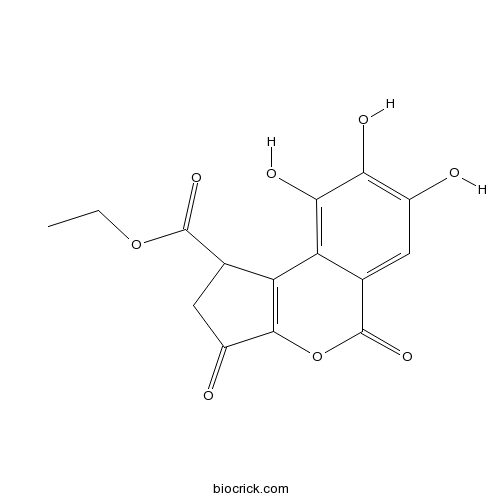
| Formula | C15H12O8 | M.Wt | 320.25 |
| Type of Compound | Phenols | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | ethyl 7,8,9-trihydroxy-3,5-dioxo-1,2-dihydrocyclopenta[c]isochromene-1-carboxylate | ||
| SMILES | CCOC(=O)C1CC(=O)C2=C1C3=C(C(=C(C=C3C(=O)O2)O)O)O | ||
| Standard InChIKey | JSEPSLOCPQODTM-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C15H12O8/c1-2-22-14(20)6-4-8(17)13-10(6)9-5(15(21)23-13)3-7(16)11(18)12(9)19/h3,6,16,18-19H,2,4H2,1H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Ethyl brevifolincarboxylate Dilution Calculator

Ethyl brevifolincarboxylate Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.1226 mL | 15.6128 mL | 31.2256 mL | 62.4512 mL | 78.064 mL |
| 5 mM | 0.6245 mL | 3.1226 mL | 6.2451 mL | 12.4902 mL | 15.6128 mL |
| 10 mM | 0.3123 mL | 1.5613 mL | 3.1226 mL | 6.2451 mL | 7.8064 mL |
| 50 mM | 0.0625 mL | 0.3123 mL | 0.6245 mL | 1.249 mL | 1.5613 mL |
| 100 mM | 0.0312 mL | 0.1561 mL | 0.3123 mL | 0.6245 mL | 0.7806 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Ethyl (9Z,11E)-13-hydroxyoctadeca-9,11-dienoate
Catalog No.:BCN9361
CAS No.:947529-92-2
- 6,6'-Di-O-sinapoylsucrose
Catalog No.:BCN9360
CAS No.:1068661-35-7
- Betulinic acid palmitate
Catalog No.:BCN9359
CAS No.:19833-15-9
- Juncusol 2-O-glucoside
Catalog No.:BCN9358
CAS No.:175094-14-1
- Convallagenin B
Catalog No.:BCN9357
CAS No.:17934-59-7
- 3-Hydroxyperillaldehyde
Catalog No.:BCN9356
CAS No.:1932348-11-2
- Methyl lucidenate A
Catalog No.:BCN9355
CAS No.:105742-79-8
- 1-[2-Hydroxy-4-(hydroxymethyl)phenyl]ethanone
Catalog No.:BCN9354
CAS No.:22518-00-9
- Methyl lucidenate D
Catalog No.:BCN9353
CAS No.:98665-09-9
- Pomegralignan
Catalog No.:BCN9352
CAS No.:1562492-32-3
- Benzyl [5-O-benzoyl-β-D-apiofuranosyl(1→2)]-β-D-glucopyranoside
Catalog No.:BCN9351
CAS No.:1097040-08-8
- Girinimbine
Catalog No.:BCN9350
CAS No.:23095-44-5
- (7R,8R)-Dihydrodehydrodiconiferyl alcohol 9-O-β-D-glucoside
Catalog No.:BCN9363
CAS No.:351346-10-6
- Pseudocolumbamine
Catalog No.:BCN9364
CAS No.:64191-04-4
- Mahanimbilol
Catalog No.:BCN9365
CAS No.:77156-13-9
- cis-Martynoside
Catalog No.:BCN9366
CAS No.:155899-92-6
- N-Phenethylcinnamamide
Catalog No.:BCN9367
CAS No.:103188-43-8
- Nandazurine
Catalog No.:BCN9368
CAS No.:49679-20-1
- Gnetifolin E
Catalog No.:BCN9369
CAS No.:140671-07-4
- (-)-Phaselic acid
Catalog No.:BCN9370
CAS No.:423170-79-0
- 16,25-Di-O-acetylcucurbitacin F
Catalog No.:BCN9371
CAS No.:2062685-10-1
- Hemslecin D
Catalog No.:BCN9372
CAS No.:586960-44-3
- (6R,9S)-3-Oxo-α-ionol glucoside
Catalog No.:BCN9373
CAS No.:159813-37-3
- 2',6'-Dihydroxy-4'-methoxy-3'-methylacetophenone
Catalog No.:BCN9374
CAS No.:69480-06-4
Bioguided Fraction and Isolation of the Antitumor Components from Phyllanthus niruri L.[Pubmed:27777954]
Biomed Res Int. 2016;2016:9729275.
Phyllanthus niruri L., a well-known medicinal plant, has been used as a folk antitumor remedy in the worldwide scale. However, the antitumor components in P. niruri have not been reported. In order to verify the antitumor components of P. niruri and the plants which have the high content of these components, we isolated the antitumor components with bioguided fraction and isolation, by different chromatographic methods from the ethyl acetate fraction of P. niruri., and identified them as Ethyl brevifolincarboxylate and corilagin by (1)H-NMR, (13)C-NMR, 2D-NMR, and mass spectrometric analyses. Cell cytotoxicity assays showed that corilagin has broad-spectrum antitumor activity, a better antitumor potential, and lower toxicity in normal cells. Besides, the coefficient of drug interaction (CDI) of 10 muM corilagin and 20 muM cDDP reached up to 0.77, which means corilagin can promote the antitumor activity of cDDP. Furthermore, by the extensive screening among 10 species of plants reported to contain corilagin, we found that Dimocarpus longan Lour. has the maximum content of corilagin. In conclusion, corilagin is the major active antitumor composition in P. niruri. L. on HCC cells and has high content in D. longan.
Constituents of the flowers of Punica granatum.[Pubmed:16887296]
Fitoterapia. 2006 Dec;77(7-8):534-7.
A new polyphenol compound named pomegranatate (1), together with, ellagic acid, 3,3',4'-tri-O-methylellagic acid, Ethyl brevifolincarboxylate, urolic and maslinic acids, and daucosterol were isolated from the ethanolic extract of the flowers of Punica granatum. The structure of compound 1 was determined by spectroscopic analysis. Maslinic acid exhibited antioxidant activity, evaluated by measurement of LDL susceptibility to oxidation.


