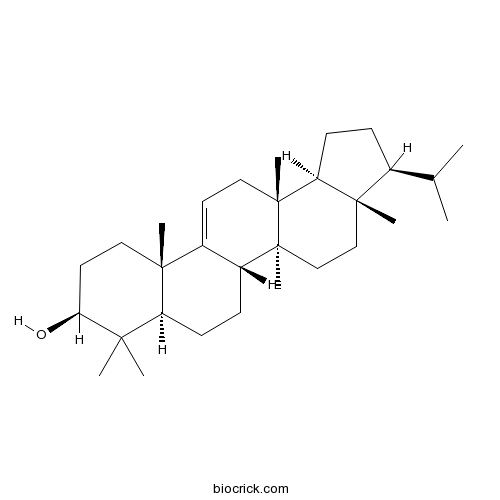
IsoarborinolCAS# 5532-41-2 |
- Sorghumol
Catalog No.:BCN4447
CAS No.:90582-44-8
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 5532-41-2 | SDF | Download SDF |
| PubChem ID | 12305182 | Appearance | Powder |
| Formula | C30H50O | M.Wt | 426.7 |
| Type of Compound | Triterpenoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | (3S,3aS,5aS,5bS,7aR,9S,11aS,13aR,13bS)-3a,5a,8,8,11a,13a-hexamethyl-3-propan-2-yl-1,2,3,4,5,5b,6,7,7a,9,10,11,13,13b-tetradecahydrocyclopenta[a]chrysen-9-ol | ||
| SMILES | CC(C)C1CCC2C1(CCC3(C2(CC=C4C3CCC5C4(CCC(C5(C)C)O)C)C)C)C | ||
| Standard InChIKey | VWYANPOOORUCFJ-MBVQSDBHSA-N | ||
| Standard InChI | InChI=1S/C30H50O/c1-19(2)20-9-12-24-28(20,6)17-18-29(7)22-10-11-23-26(3,4)25(31)14-15-27(23,5)21(22)13-16-30(24,29)8/h13,19-20,22-25,31H,9-12,14-18H2,1-8H3/t20-,22+,23-,24-,25-,27+,28-,29-,30+/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Isoarborinol Dilution Calculator

Isoarborinol Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.3436 mL | 11.7178 mL | 23.4357 mL | 46.8713 mL | 58.5892 mL |
| 5 mM | 0.4687 mL | 2.3436 mL | 4.6871 mL | 9.3743 mL | 11.7178 mL |
| 10 mM | 0.2344 mL | 1.1718 mL | 2.3436 mL | 4.6871 mL | 5.8589 mL |
| 50 mM | 0.0469 mL | 0.2344 mL | 0.4687 mL | 0.9374 mL | 1.1718 mL |
| 100 mM | 0.0234 mL | 0.1172 mL | 0.2344 mL | 0.4687 mL | 0.5859 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Rubuminatus B
Catalog No.:BCN9151
CAS No.:1772614-25-1
- (3,4-Dihydroxy-5-methoxybenzoyl)taraxerol
Catalog No.:BCN9150
CAS No.:2241135-31-7
- Stephalonine M
Catalog No.:BCN9149
CAS No.:2376321-05-8
- cis-Nerolidol
Catalog No.:BCN9148
CAS No.:3790-78-1
- Stephalonine P
Catalog No.:BCN9147
CAS No.:2376309-57-6
- Stephalonine N
Catalog No.:BCN9146
CAS No.:2376321-20-7
- Stephalonine L
Catalog No.:BCN9145
CAS No.:2379277-60-6
- 3,4-Dihydroxybenzoyllupeol
Catalog No.:BCN9144
CAS No.:2231323-99-0
- 6-(3-Methyl-2-oxobutyroyl)-7-methoxycoumarin
Catalog No.:BCN9143
CAS No.:2188162-96-9
- Epirengynic acid
Catalog No.:BCN9142
CAS No.:1310146-00-9
- 6′′-O-β-D-Apiofuranosylapterin
Catalog No.:BCN9141
CAS No.:2188162-94-7
- Sinapoyl sinapaldehyde
Catalog No.:BCN9140
CAS No.:
- Isoarborinol acetate
Catalog No.:BCN9153
CAS No.:5595-78-8
- Glabralide B
Catalog No.:BCN9154
CAS No.:2170388-84-6
- Glabralide C
Catalog No.:BCN9155
CAS No.:2170388-85-7
- Myricadenin A
Catalog No.:BCN9156
CAS No.:1612239-23-2
- Fuscaxanthone A
Catalog No.:BCN9157
CAS No.:499777-91-2
- Glabralide A
Catalog No.:BCN9158
CAS No.:1969289-10-8
-
(-)-Evofolin B
Catalog No.:BCN9159
CAS No.:1961305-60-1
- Soyasapogenol A 21-O-α-L-rhamnopyranoside
Catalog No.:BCN9160
CAS No.:2067321-88-2
- (11Bs)-7,11-dihydroxy-3,4,9,11b-tetramethyl-1,2,8,9-tetrahydronaphtho[2,1-f][1]benzofuran-6-one
Catalog No.:BCN9161
CAS No.:
- 6-Hydroxycoumurrayin
Catalog No.:BCN9162
CAS No.:2188162-97-0
- Prionidipene A
Catalog No.:BCN9163
CAS No.:2199455-72-4
- 4,18-Dihydro-4-hydroxysaprirearine
Catalog No.:BCN9164
CAS No.:2202760-88-9
Antiamoebic Activity of Petiveria alliacea Leaves and Their Main Component, Isoarborinol.[Pubmed:28621111]
J Microbiol Biotechnol. 2017 Aug 28;27(8):1401-1408.
Petiveria alliacea L. (Phytolacaceae) is a medicinal plant with a broad range of traditional therapeutic properties, including the treatment of dysentery and intestinal infections caused by protozoan parasites. However, its effects against Entamoeba histolytica have not been reported yet. We investigated the antiamoebic activity present in the leaves of P. alliacea Antiamoebic activity was evaluated in methanolic and aqueous extracts, as well as in the hexanic, methanolic, and EtOAc fractions. The P. alliacea methanolic extract showed a better antiamoebic activity than the aqueous extract with an IC50 = 0.51 mg/ml. Likewise, the hexanic fraction was the most effective fraction, showing a dose-dependent activity against E. histolytica, with an IC50 = 0.68 mg/ml. Hexanic subfraction 12-19 showed the highest antiamoebic activity at 0.8 mg/ml, producing 74.3% growth inhibition without any toxicity in mammal cells. A major component in subfraction 12-19 was identified as Isoarborinol, which produced 51.4% E. histolytica growth inhibition at 0.05 mg/ml without affecting mammal cells. The P. alliacea leaf extract has antiamoebic activity that can be attributed to a major metabolite known as Isoarborinol.
Synthesis of arborane triterpenols by a bacterial oxidosqualene cyclase.[Pubmed:28028245]
Proc Natl Acad Sci U S A. 2017 Jan 10;114(2):245-250.
Cyclic triterpenoids are a broad class of polycyclic lipids produced by bacteria and eukaryotes. They are biologically relevant for their roles in cellular physiology, including membrane structure and function, and biochemically relevant for their exquisite enzymatic cyclization mechanism. Cyclic triterpenoids are also geobiologically significant as they are readily preserved in sediments and are used as biomarkers for ancient life throughout Earth's history. Isoarborinol is one such triterpenoid whose only known biological sources are certain angiosperms and whose diagenetic derivatives (arboranes) are often used as indicators of terrestrial input into aquatic environments. However, the occurrence of arborane biomarkers in Permian and Triassic sediments, which predates the accepted origin of angiosperms, suggests that microbial sources of these lipids may also exist. In this study, we identify two Isoarborinol-like lipids, eudoraenol and adriaticol, produced by the aerobic marine heterotrophic bacterium Eudoraea adriatica Phylogenetic analysis demonstrates that the E. adriatica eudoraenol synthase is an oxidosqualene cyclase homologous to bacterial lanosterol synthases and distinct from plant triterpenoid synthases. Using an Escherichia coli heterologous sterol expression system, we demonstrate that substitution of four amino acid residues in a bacterial lanosterol synthase enabled synthesis of pentacyclic arborinols in addition to tetracyclic sterols. This variant provides valuable mechanistic insight into triterpenoid synthesis and reveals diagnostic amino acid residues to differentiate between sterol and arborinol synthases in genomic and metagenomic datasets. Our data suggest that there may be additional bacterial arborinol producers in marine and freshwater environments that could expand our understanding of these geologically informative lipids.
Ethnobotany, phytochemistry and neuropharmacological effects of Petiveria alliacea L. (Phytolaccaceae): A review.[Pubmed:26944236]
J Ethnopharmacol. 2016 Jun 5;185:182-201.
ETHNOPHARMACOLOGICAL RELEVANCE: Petiveria alliacea L. commonly grows in the tropical regions of the Americas such as the Amazon forest, Central America, Caribbean islands and Mexico, as well as specific regions of Africa. Popularly known by several different names including 'mucuracaa', 'guine' and 'pipi', P. alliacea has been used in traditional medicine for the treatment of various central nervous system (CNS) disorders, such as anxiety, pain, memory deficits and seizures, as well as for its anaesthetic and sedative properties. Furthermore, the use of this species for religious ceremonies has been reported since the era of slavery in the Americas. Therefore, the present review aims to provide a critical and comprehensive overview of the ethnobotany, phytochemistry and pharmacological properties of P. alliacea, focusing on CNS pharmacological effects, in order to identify scientific lacunae and to open new perspectives for future research. MATERIALS AND METHODS: A literature search was performed on P. alliacea using ethnobotanical textbooks, published articles in peer-reviewed journals, unpublished materials, government survey reports and scientific databases such as PubMed, Scopus, Web of Science, Science Direct and Google Scholar. The Plant List, International Plant Name Index and Kew Botanical Garden Plant name databases were used to validate the scientific names. RESULTS AND DISCUSSION: Crude extracts, fractions and phytochemical constituents isolated from various parts of P. alliacea show a wide spectrum of neuropharmacological activities including anxiolytic, antidepressant, antinociceptive and anti-seizure, and as cognitive enhancers. Phytochemistry studies of P. alliacea indicate that this plant contains a diversity of biologically active compounds, with qualitative and quantitative variations of the major compounds depending on the region of collection and the harvest season, such as essential oil (Petiverina), saponinic glycosides, Isoarborinol-triterpene, Isoarborinol-acetate, Isoarborinol-cinnamate, steroids, alkaloids, flavonoids and tannins. Root chemical analyses have revealed coumarins, benzyl-hydroxy-ethyl-trisulphide, benzaldehyde, benzoic acid, dibenzyl trisulphide, potassium nitrate, b-sitosterol, Isoarborinol, Isoarborinol-acetate, Isoarborinol-cinnamate, polyphenols, trithiolaniacine, glucose and glycine. CONCLUSIONS: Many traditional uses of P. alliacea have now been validated by modern pharmacology research. The available data reviewed here support the emergence of P. alliacea as a potential source for the treatment of different CNS disorders including anxiety, depression, pain, epilepsy and memory impairments. However, further studies are certainly required to improve the knowledge about the mechanisms of action, toxicity and efficacy of the plant as well as about its bioactive compounds before it can be approved in terms of its safety for therapeutic applications.
Hybanthus enneaspermus (L.) F. Muell: a concise report on its phytopharmacological aspects.[Pubmed:23725830]
Chin J Nat Med. 2013 May;11(3):199-206.
Hybanthus enneaspermus (L.) F. Muell belonging to the family Violaceae, popularly known as Ratanpurus (Hindi) is a herb or a shrub distributed in the tropical and subtropical regions of the world. In the Ayurvedic literature, the plant is reported to cure conditions of "Kapha" and "Pitta", urinary calculi, strangury, painful dysentery, vomiting, burning sensation, wandering of the mind, urethral discharge, blood trouble, asthma, epilepsy, cough, and to give tone to the breasts. Phytochemically, the plant contains a considerable amount of dipeptide alkaloids, aurantiamide acetate, Isoarborinol, and beta-sitosterol, sugars, flavonoids, steroids, triterpenes, phenols, flavones, catachins, tannins, anthraquinones and amino acids. Pharmacologically, the plant is reported to possess antidiabetic, antiplasmodial, antimicrobial, anticonvulsant, nephroprotective, aphrodisiac, hepatoprotective, antiinflammatory, aldose reductase inhibitory and free radical scavenging activities. The information provided in this review will be worthwhile to know the applicability of H. enneaspermus for the treatment of various acute or chronic diseases with a diverse nature of phytoconstituents. The overall data in this review article were collected from various scientific sources on the research of H. enneaspermus.
Divergent evolution of oxidosqualene cyclases in plants.[Pubmed:22150097]
New Phytol. 2012 Mar;193(4):1022-38.
Triterpenes are one of the largest classes of plant metabolites and have important functions. A diverse array of triterpenoid skeletons are synthesized via the isoprenoid pathway by enzymatic cyclization of 2,3-oxidosqualene. The genomes of the lower plants Chlamydomonas reinhardtii and moss (Physcomitrella patens) contain just one oxidosqualene cyclase (OSC) gene (for sterol biosynthesis), whereas the genomes of higher plants contain nine to 16 OSC genes. Here we carry out functional analysis of rice OSCs and rigorous phylogenetic analysis of 96 OSCs from higher plants, including Arabidopsis thaliana, Oryza sativa, Sorghum bicolor and Brachypodium distachyon. The functional analysis identified an amino acid sequence for Isoarborinol synthase (OsIAS) (encoded by Os11g35710/OsOSC11) in rice. Our phylogenetic analysis suggests that expansion of OSC members in higher plants has occurred mainly through tandem duplication followed by positive selection and diversifying evolution, and consolidated the previous suggestion that dicot triterpene synthases have been derived from an ancestral lanosterol synthase instead of directly from their cycloartenol synthases. The phylogenetic trees are consistent with the reaction mechanisms of the protosteryl and dammarenyl cations which parent a wide variety of triterpene skeletal types, allowing us to predict the functions of the uncharacterized OSCs.
Bioactive triterpenes from Diospyros blancoi.[Pubmed:19731144]
Nat Prod Res. 2009;23(13):1252-8.
The ethyl acetate extract of the air-dried leaves of Diospyros blancoi afforded Isoarborinol methyl ether (1), a mixture of alpha-amyrin palmitate, alpha-amyrin palmitoleate, beta-amyrin palmitate and beta-amyrin palmitoleate (2) in a 13 : 4 : 3 : 1 ratio, and squalene. The structures of 1, 2 and squalene were elucidated by extensive 1D and 2D NMR spectroscopy. Compounds 1 and 2 exhibited antimicrobial activity against Escherichia coli, Pseudomonas aeruginosa, Candida albicans, Staphylococcus aureus and Trichophyton mentagrophytes, and were found inactive against Bacillus subtilis and Aspergillus niger. Sample 2 exhibited significant analgaesic and anti-inflammatory activities.
[Studies on the chemical constituents from the aerial parts of Breynia fruticosa].[Pubmed:15656136]
Zhongguo Zhong Yao Za Zhi. 2004 Nov;29(11):1052-4.
OBJECTIVE: To investigate the chemical constituents from the aerial parts of Breynia fruticosa. METHOD: Various chromatographic techniques were employed for isolation and purification of the constituents. The structures were elucidated by chemical evidence and spectral methods. RESULT: Seven compounds were obtained and identified by spectroscopic methods and compared with authentic samples as aviculin [(+)-isolariciresinol-9'-rhamno-pyranoside], friedelan-3beta-ol, friedelin, arborinone, Isoarborinol, 5-hydroxy-7,8,4'-trimethoxy flavone, 2,4-dihydroxy-6-methoxy-3-methyl-acetophenone. CONCLUSION: All compounds were firstly isolated from B. genus, furthermore, aviculin was isolated from Euphorbiaceae for the first time.
[Studies on the chemical constituents from the roots and rhizomes of Gerbera piloselloides].[Pubmed:12776495]
Zhongguo Zhong Yao Za Zhi. 2002 Aug;27(8):594-6.
OBJECTIVE: To study the chemical constituents from the roots and rhizomes of Gerbera piloselloides. METHOD: The compounds were isolated by silica gel column chromatography, and their structures were elucidated by means of spectral analysis. RESULT: Seven compounds were identified as bothrioclinin (I), hydroquinone (II), marmesin (III), succinic acid (IV), umbelliferone (V), Isoarborinol (VI) and beta-sitosterol (VII). CONCLUSION: Compound I, V and VI were isolated from Gerbera genus for the first time.
Two novel flavanones from Greigia sphacelata.[Pubmed:11141119]
J Nat Prod. 2000 Dec;63(12):1689-91.
As part of our continuing phytochemical investigations of plants from arid environments in Chile, the aerial parts of Greigia sphacelata were examined. Two novel flavanones, 5,7,3'-trihydroxy-6, 4',5'-trimethoxyflavanone (1) and 5,3'-dihydroxy-6,7,4', 5'-tetramethoxyflavanone (2), as well as eight known compounds-1, 3-O-di-trans-p-coumaroylglycerol (3), 1-O-trans-p-coumaroylglycerol (4), a mixture of 1-(omega-feruloyldocosanoyl)glycerol (5) and 1-(omega-feruloyltetracosanoyl)glycerol (6), trans-ferulic acid 22-hydroxydocosanoic acid ester (7), arborinone (8), arborinol (9), and Isoarborinol (10)-were isolated.
A new 3,4-seco-ursane triterpenoid from Glyptopetalum sclerocarpum.[Pubmed:10993235]
Chem Pharm Bull (Tokyo). 2000 Sep;48(9):1347-9.
A new ursane-type triterpene, glyptopetolide, was isolated along with two known triterpenoids, Isoarborinol and cangoronine, from the stem bark of Glyptopetalum sclerocarpum LAWS. (Celastraceae). The structure of glyptopetolide was elucidated as 3,4-seco-14alpha,27-cyclo-urs-4(23)-en-3,11alpha-ol ide by spectroscopic analysis.
The interaction of various cholesterol 'ancestors' with lipid membranes: a 2H-NMR study on oriented bilayers.[Pubmed:1586660]
Biochim Biophys Acta. 1992 Apr 13;1105(2):213-20.
The effect of putative cholesterol 'precursors' on model membranes has been studied by deuterium nuclear, magnetic resonance (2H-NMR) spectroscopy. Oriented bilayers were prepared from 1-myristoyl-2-[2H27 myristoyl-sn-glycero-3-phosphocholine (DMPC-d27) and tricyclohexaprenols or octaprenediols. Order parameter profiles were determined and showed that tricyclohexaprenols and octaprenediols increase the acyl chain order in DMPC bilayers, but to a smaller extent than cholesterol. The order parameter increases, depending on the chain position, from 5% to 7% in the presence of ditertiary octaprenediol, and from 16% to 21% in the presence of tricyclohexaprenol-Z,Z. Aqueous multilamellar dispersions of DMPC-d27 and of DMPC-d27 containing 30 mol% tricyclohexaprenol-E,E were prepared, and the first moments calculated from 2H-NMR spectra over the temperature range 5-55 degrees C. Tricyclohexaprenol-E,E almost abolishes the phase transition of DMPC. Thus, as predicted, tricyclohexaprenols and octaprenediols have a cholesterol-like behaviour in lipid membranes; however their effect on the model DMPC system is weak. On the contrary, Isoarborinol has no effect on the lipid chain order in the liquid-crystalline phase of DMPC bilayers. 2H-NMR spectra of aqueous dispersions of DMPC-d27 and 30 mol% Isoarborinol between 25 and 60 degrees C showed the coexistence of two lamellar phases over a wide temperature range, which was confirmed by differential scanning calorimetry (DSC) and 31P-NMR spectroscopy. This absence of ordering effect of Isoarborinol might be related to some inherent structural features.
Triterpene alcohol isolation from oil shale.[Pubmed:17847547]
Science. 1969 Mar 14;163(3872):1192-3.
Isoarborinol, an intact pentacyclic unsaturated alcohol, was isolated from the Messel oil shale (about 50 x 106 years old). Complex organic substances, even those very sensitive to oxidation, reduction, or acidic conditions, can thus survive without alteration for long periods.


