Leonuriside ACAS# 121748-12-7 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 121748-12-7 | SDF | Download SDF |
| PubChem ID | 14237626 | Appearance | Powder |
| Formula | C14H20O9 | M.Wt | 332.30 |
| Type of Compound | Phenols | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
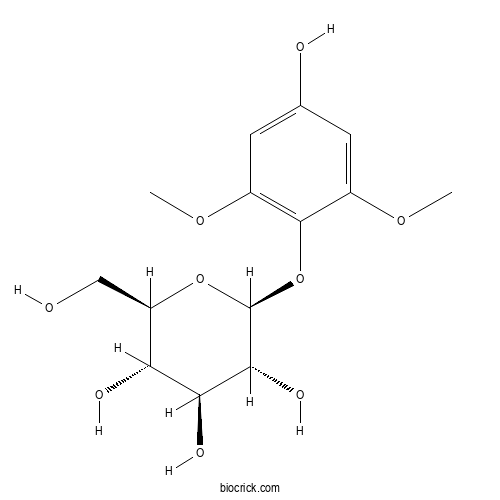
| Chemical Name | (2S,3R,4S,5S,6R)-2-(4-hydroxy-2,6-dimethoxyphenoxy)-6-(hydroxymethyl)oxane-3,4,5-triol | ||
| SMILES | COC1=CC(=CC(=C1OC2C(C(C(C(O2)CO)O)O)O)OC)O | ||
| Standard InChIKey | NOQYJICHFNSIFZ-DIACKHNESA-N | ||
| Standard InChI | InChI=1S/C14H20O9/c1-20-7-3-6(16)4-8(21-2)13(7)23-14-12(19)11(18)10(17)9(5-15)22-14/h3-4,9-12,14-19H,5H2,1-2H3/t9-,10-,11+,12-,14+/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Leonuriside A Dilution Calculator

Leonuriside A Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.0093 mL | 15.0466 mL | 30.0933 mL | 60.1866 mL | 75.2332 mL |
| 5 mM | 0.6019 mL | 3.0093 mL | 6.0187 mL | 12.0373 mL | 15.0466 mL |
| 10 mM | 0.3009 mL | 1.5047 mL | 3.0093 mL | 6.0187 mL | 7.5233 mL |
| 50 mM | 0.0602 mL | 0.3009 mL | 0.6019 mL | 1.2037 mL | 1.5047 mL |
| 100 mM | 0.0301 mL | 0.1505 mL | 0.3009 mL | 0.6019 mL | 0.7523 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- threo-Guaiacylglycerol β-dihydroconiferyl ether
Catalog No.:BCX0169
CAS No.:135820-78-9
- Justicidin A
Catalog No.:BCX0168
CAS No.:25001-57-4
- 3alpha-Acetyloxy-25-hydroxyolean-12-en-28-oic acid
Catalog No.:BCX0167
CAS No.:469895-08-7
- Pyrolone B
Catalog No.:BCX0166
CAS No.:1014978-30-3
- Gerardianin D
Catalog No.:BCX0165
CAS No.:2036276-69-2
- Retrofractamide C
Catalog No.:BCX0164
CAS No.:96386-33-3
- Justicidinoside C
Catalog No.:BCX0163
CAS No.:177912-23-1
- Massonianoside A
Catalog No.:BCX0162
CAS No.:623945-11-9
- Justicidin C
Catalog No.:BCX0161
CAS No.:17803-12-2
- Lapathoside A
Catalog No.:BCX0160
CAS No.:373646-49-2
- Kaempferol 3-(2''-galloylglucoside)
Catalog No.:BCX0159
CAS No.:76343-90-3
- Procumbenoside E
Catalog No.:BCX0158
CAS No.:220182-12-7
- (+)-3-Hydroxy-p-menth-1-en-6-one
Catalog No.:BCX0171
CAS No.:120850-08-0
- (-)-Byakangelicin
Catalog No.:BCX0172
CAS No.:23517-02-4
- 5,5'-Oxybis(pyrrolidin-2-one)
Catalog No.:BCX0173
CAS No.:73123-79-2
- Macarangin
Catalog No.:BCX0174
CAS No.:129385-65-5
- 3-(4-Hydroxy-3-methoxyphenyl)propane-1,2-diol
Catalog No.:BCX0175
CAS No.:27391-18-0
- Anthriscifoldine B
Catalog No.:BCX0176
CAS No.:1158213-52-5
- α-Cembrenediol
Catalog No.:BCX0177
CAS No.:57605-80-8
- β-Cembrenediol
Catalog No.:BCX0178
CAS No.:57605-81-9
- Vesuvianic acid
Catalog No.:BCX0179
CAS No.:63090-99-3
- N-Tetracosanoyltyramine
Catalog No.:BCX0180
CAS No.:113122-70-6
- Lycoctonine
Catalog No.:BCX0181
CAS No.:26000-17-9
- 2',4',6'-Trihydroxydihydrochalcone
Catalog No.:BCX0182
CAS No.:1088-08-0
[A new sesquiterpenoid from Lindera aggregata].[Pubmed:38621903]
Zhongguo Zhong Yao Za Zhi. 2024 Feb;49(4):961-967.
The chemical composition of the aqueous part of the extract from Lindera aggregata was studied, which was separated and purified by the macroporous resin column chromatography, MCI medium pressure column chromatography, semi-preparative high-performance liquid phase and other methods. The structures of the compounds were identified according to physical and chemical properties and spectroscopic data. Thirteen compounds were isolated and identified from the aqueous extracts, which were identified as(1S,3R,5R,6R,8S,10S)-epi-lindenanolide H(1), tachioside(2), lindenanolide H(3), Leonuriside A(4), 3,4-dihydroxyphenyl ethyl beta-D-glucopyranoside(5), 3,4,5-trimethoxyphenol-1-O-6-alpha-L-rhamnose-(1-->6)-O-beta-D-glucoside(6), 5-hydroxymethylfurfural(7),(+)-lyoniresin-4-yl-beta-D-glucopyranoside(8), lyoniside(9), norboldine(10), norisopordine(11), boldine(12), reticuline(13). Among them, compound 1 was a new one, and compounds 2, 5, 6, 8, 9 were obtained from L. aggregata for the first time. The inflammatory model was induced by lipopolysaccharide(LPS) in the RAW264.7 cells. The results showed that compounds 1, 8, 10 and 12 had significant anti-inflammatory activity.
Ethnobotany and phytochemistry of plants used to treat musculoskeletal disorders among Skaw Karen, Thailand.[Pubmed:38131672]
Pharm Biol. 2024 Dec;62(1):62-104.
CONTEXT: Musculoskeletal system disorders (MSD) are prevalent around the world affecting the health of people, especially farmers who work hard in the field. Karen farmers use many medicinal plants to treat MSD. OBJECTIVE: This study collects traditional plant-based remedies used by the Skaw Karen to treat MSD and evaluates their active phytochemical compounds. MATERIALS AND METHODS: The ethnobotanical study was conducted in six Karen villages in Chiang Mai province using semi-structured interviews were of 120 informants. The data were analyzed using ethnobotanical indices including use values (UV), choice value (CV), and informant consensus factor (ICF). Consequently, the 20 most important species, according to the indices, were selected for phytochemical analysis using LC-MS/MS. RESULTS: A total of 3731 use reports were obtained for 139 species used in MSD treatment. The most common ailments treated with those plants were muscular pain. A total of 172 high-potential active compounds for MSD treatment were identified. Most of them were flavonoids, terpenoids, alkaloids, and steroids. The prevalent phytochemical compounds related to treat MSD were 9-hydroxycalabaxanthone, dihydrovaltrate, morroniside, isoacteoside, lithocholic acid, pomiferin, cucurbitacin E, Leonuriside A, liriodendrin, and physalin E. Sambucus javanica Reinw. ex Blume (Adoxaceae), Betula alnoides Buch.-Ham. ex D.Don (Betulaceae), Blumea balsamifera (L.) DC. (Asteraceae), Plantago major L. (Plantaginaceae) and Flacourtia jangomas (Lour.) Raeusch. (Salicaceae) all had high ethnobotanical index values and many active compounds. DISCUSSION AND CONCLUSIONS: This study provides valuable information, demonstrating low-cost medicine plants that are locally available. It is a choice of treatment for people living in remote areas.
Characterization of Ikaria Heather Honey by Untargeted Ultrahigh-Performance Liquid Chromatography-High Resolution Mass Spectrometry Metabolomics and Melissopalynological Analysis.[Pubmed:35936100]
Front Chem. 2022 Jul 22;10:924881.
Honey represents a valuable food commodity, known since ancient times for its delicate taste and health benefits due to its specific compositional characteristics, mainly the phenolic compound content. "Anama" honey is a monofloral honey produced from the nectar of Erica manipuliflora plant, a heather bush of the Greek island of Ikaria, one of the Mediterranean's longevity regions. "Anama" is characterized by a unique aroma and taste, with a growing demand for consumption and the potential to be included in the list of products with a protected designation of origin. The aim of this study was to determine the chemical and botanical profile of authentic Anama honey samples and find similarities and differences with honey samples of a different botanical origin from the same geographical area. Untargeted Ultrahigh-Performance Liquid Chromatography-Hybrid Quadrupole-Orbitrap High-Resolution Mass Spectrometry (UHPLC-HRMS) metabolomics study was conducted on authentic heather, pine, and thyme honey samples from Ikaria and neighboring islands. The Principal Component Analysis (PCA), Orthogonal Projections to Latent Structures Discriminant Analysis (OPLS-DA), and differential analysis were performed using the entire metabolic profile of the samples and allowed the identification of chemical markers for sample discrimination. Thirty-two characteristic secondary metabolites (cinnamic acids, phenolic acids, flavonoids, terpenes) and other bioactive phenolic compounds, some of them not previously reported in a heather honey (aucubin, catalpol, domesticoside, Leonuriside A, picein among others), emerged as potential chemical indicators of Anama honey. Melissopalynological analysis was also carried out to decipher the botanical and geographical origin of Anama honey. The relative frequency of the pollen of dominant plants of the Ericaceae family and a multitude of nectariferous and nectarless plants contributing to the botanical profile of Anama was evaluated. The identification of the pollen sources enabled a potential correlation of differentially increased secondary metabolites and chemicals with their botanical origin. The physicochemical profile of Anama was also determined, including the parameters of pH, color, electrical conductivity, diastase, moisture, as well as sugars, supporting the high quality of this heather honey.
ytotoxic Constituents of Mallotus microcarpus.[Pubmed:30549897]
Nat Prod Commun. 2017 Mar;12(3):407-408.
A new 3-methoxybenzensulfonic acid 4-0-0-D-glucopyranoside (1), and ten known compounds (2-11) were isolated from the methanolic extract of the stems of Mallotus microcarpus. The cytotoxicity of the isolated compounds was evaluated by the MTT method. 3-Methoxybenzensulfonic acid 4-Omicron-beta-D- glucopyranoside (1) and methyl salicylate 2-rutinoside (5) showed strong cytotoxicity against EGFR-TKI-resistant human lung cancer A549 cells in comparison with camptothecin. Compound 1, Leonuriside A (2), 3,4'-dihydroxypropiophenone 3-Omicron-glucoside (6) and (lR,2S)-hovetrichoside A (10) inhibited the growth of human breast cancer MCF-7 cell line with IC(5)(0) values in the range of 0.48-1.78 muM. This is the first report on the chemical composition and cytotoxic activity of M microcarpus.
Phytochemical investigations of Lonchocarpus bark extracts from Monteverde, Costa Rica.[Pubmed:24868870]
Nat Prod Commun. 2014 Apr;9(4):507-10.
The acetone bark extracts of three species of Lonchocarpus from Monteverde, Costa Rica, L. atropurpureus, L. oliganthus, and L. monteviridis, were screened for antibacterial, cytotoxic, and antioxidant activities. L. orotinus extract was antibacterial against Bacillus cereus (MIC = 39 microg/mL), while L. monteviridis exhibited the most antioxidant activity. None of the Lonchocarpus extracts showed cytotoxic activity against MCF-7 cells. Fatty acids and atraric acid were isolated and purified from L. atropurpureus bark, fatty acids and loliolide from L. oliganthus bark, and Leonuriside A and beta-D-glucopyranos-1-yl N-methylpyrrole-2-carboxylate from L. monteviridis bark. Atraric acid showed cytotoxic and antimicrobial activities.
Phenolic components from Rhus parviflora fruits and their inhibitory effects on lipopolysaccharide-induced nitric oxide production in RAW 264.7 macrophages.[Pubmed:23822726]
Nat Prod Res. 2013;27(23):2244-7.
Nine phenolic compounds, phloracetophenone-4-O-beta-D-glucopyranoside (1), p-hydroxybenzoic acid-4-O-beta-D-glucopyranoside (2), Leonuriside A (3), 3-methoxy-4-hydroxyphenol-1-O-beta-D-glucopyranoside (4), cis-p-coumaric acid-4-O-beta-D-glucopyranoside (5), trans-p-coumaric acid-4-O-beta-D-glucopyranoside (6), trans-p-coumaric acid-9-O-beta-D-glucopyranoside (7), (-)-shikimic acid (8) and (-)-methyl shikimate (9), were isolated for the first time from the fruits of Rhus parviflora. Compounds 1, 3-6 and 8 inhibited lipopolysaccharide-stimulated nitric oxide (NO) production and inducible NO synthase expression in RAW 264.7 macrophages with IC50 values of 9.24 +/- 1.20, 21.37 +/- 2.02, 23.07 +/- 1.58, 9.86 +/- 0.98, 19.05 +/- 1.66 and 11.3 +/- 1.54 muM, respectively. The results indicated possible use of compounds for the treatment of inflammatory diseases.
Isolation and characterization of phenolic compounds from the leaves of Salix matsudana.[Pubmed:18794770]
Molecules. 2008 Aug 3;13(8):1530-7.
A bioassay-guided in vitro screen has revealed that a 70% methanol extract of the leaves of Salix matsudana shows considerable inhibitory activity against cyclooxygenases (COX-1 and COX-2). A subsequent phytochemical study led to the isolation of a new flavonoid, matsudone A (1), together with five known flavonoids--luteolin (2), isoquercitrin (3), 7-methoxyflavone (4), luteolin 7-O-glucoside (5), 4',7-dihydroxyflavone (6)--and two phenolic glycosides, Leonuriside A (7) and piceoside (8). Their structures were elucidated on the basis of extensive 1D- and 2D-NMR studies, high resolution ESI mass spectroscopic analyses and comparisons with literature data. The isolated compounds 1-8 were tested for their inhibitory activities against COX-1 and COX-2. Compounds 1, 5 and 6 were found to have potent inhibitory effect on COX-2 and compounds 3-5 exhibited moderate inhibition against COX-1.
Two new phenolic water-soluble constituents from branch bark of Davidia involucrata.[Pubmed:18415854]
Nat Prod Res. 2008 Apr 15;22(6):483-8.
Two new phenolic water-soluble constituents, involcranoside A (1) and involcranoside B (2) have been isolated along with five known phenolic compounds: 3,4-dimethoxyphenyl-O-beta-D-gluco-pyranoside (3), picein (4), and 1,4-dihydroxy-3-methoxy-phenyl-4-O-beta-D-glucopyranoside (5), Leonuriside A (6) and 4-hydroxy-3-methoxybenzoic acid (7) from the branch bark of Davidia involucrata. Identification of their structures was achieved by 1D and 2D NMR experiments, including (1)H-(1)H COSY, NOESY, HMQC and HMBC methods and FAB mass spectral data.
[Chemical constituents from Spatholobus sinensis].[Pubmed:18357735]
Yao Xue Xue Bao. 2008 Jan;43(1):67-70.
Spatholobus sinensis is a plant of the Spatholobus genus (Leguminosae family). Its caulis are used as "ji-xue-teng" regionally. However, to our knowledge, no phytochemical investigation on S. sinensis has been reported to date. In this study, eight compounds were isolated from the ethanol extract of the caulis of S. sinensis, by solvents extraction and column chromatography methods. By analysis of their physic-chemical constants and spectral data, the structures of 8 compounds were identified as spatholosineside A (1), 2',4',5,7-tetrahydroxyisoflavone (2), isoliquiritigenin (3), lupinalbin A (4), coumestrol (5), naringenin (6), protocatechuic acid (7), Leonuriside A (8). Compound 1 is a new compound.


