N-DeacetyllappaconitineCAS# 11033-64-0 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 11033-64-0 | SDF | Download SDF |
| PubChem ID | 162639976.0 | Appearance | Powder |
| Formula | C30H42N2O7 | M.Wt | 542.67 |
| Type of Compound | Alkaloids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
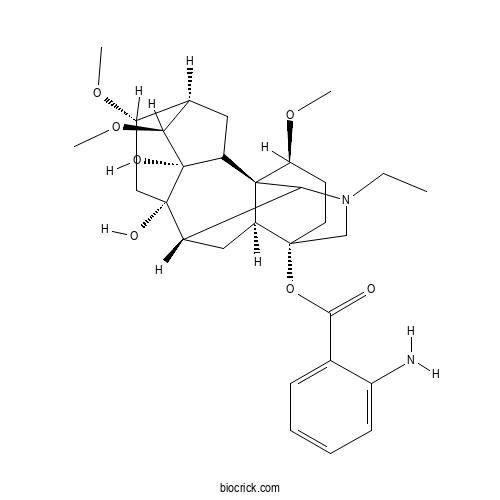
| Chemical Name | [(1S,3S,4S,5R,6S,8S,9S,13S,16S,17S)-11-ethyl-3,8-dihydroxy-4,6,16-trimethoxy-11-azahexacyclo[7.7.2.12,5.01,10.03,8.013,17]nonadecan-13-yl] 2-aminobenzoate | ||
| SMILES | CCN1CC2(CCC(C34C2CC(C31)C5(CC(C6CC4C5(C6OC)O)OC)O)OC)OC(=O)C7=CC=CC=C7N | ||
| Standard InChIKey | VSUODASNSRJNCP-BCLOKKRQSA-N | ||
| Standard InChI | InChI=1S/C30H42N2O7/c1-5-32-15-27(39-26(33)16-8-6-7-9-19(16)31)11-10-23(37-3)29-21(27)13-18(24(29)32)28(34)14-20(36-2)17-12-22(29)30(28,35)25(17)38-4/h6-9,17-18,20-25,34-35H,5,10-15,31H2,1-4H3/t17-,18+,20+,21-,22?,23+,24?,25+,27-,28+,29+,30+/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

N-Deacetyllappaconitine Dilution Calculator

N-Deacetyllappaconitine Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.8427 mL | 9.2137 mL | 18.4274 mL | 36.8548 mL | 46.0685 mL |
| 5 mM | 0.3685 mL | 1.8427 mL | 3.6855 mL | 7.371 mL | 9.2137 mL |
| 10 mM | 0.1843 mL | 0.9214 mL | 1.8427 mL | 3.6855 mL | 4.6069 mL |
| 50 mM | 0.0369 mL | 0.1843 mL | 0.3685 mL | 0.7371 mL | 0.9214 mL |
| 100 mM | 0.0184 mL | 0.0921 mL | 0.1843 mL | 0.3685 mL | 0.4607 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Ergosta-7,22,24(28)-trien-3β-ol
Catalog No.:BCX1209
CAS No.:36680-39-4
- Rhamnetin-3-O-β-D-Glucoside
Catalog No.:BCX1208
CAS No.:27875-34-9
- Crocin IV
Catalog No.:BCX1207
CAS No.:57710-64-2
- Tarasaponin VI
Catalog No.:BCX1206
CAS No.:59252-95-8
- Iodixanol Impurity C
Catalog No.:BCX1205
CAS No.:171897-74-8
- Isoproprotogracillin B
Catalog No.:BCX1204
CAS No.:117457-34-8
- 8-Keto-berberine
Catalog No.:BCX1203
CAS No.:81397-08-2
- Tigogenin lactone
Catalog No.:BCX1202
CAS No.:514-33-0
- 21-keto-gypenoside A
Catalog No.:BCX1201
CAS No.:1392136-41-2
- Alltride
Catalog No.:BCX1200
CAS No.:2050-87-5
- 4α-Phorbol
Catalog No.:BCX1199
CAS No.:26241-63-4
- Markogenin
Catalog No.:BCX1198
CAS No.:562-35-6
- Ergosterol Acetate
Catalog No.:BCX1211
CAS No.:2418-45-3
- 6''-Acetylhyperin
Catalog No.:BCX1212
CAS No.:72659-75-7
- Ethyl linoleate
Catalog No.:BCX1213
CAS No.:544-35-4
- Hydroxy-ε-sanshool
Catalog No.:BCX1214
CAS No.:252193-26-3
- Dihydrooroxylin A-7-O-β-D-glucuronide
Catalog No.:BCX1215
CAS No.:1370002-08-6
- α-D-altro-3-Heptulofuranose
Catalog No.:BCX1216
CAS No.:25545-06-6
- Linocinnamarin
Catalog No.:BCX1217
CAS No.:554-87-0
- Panaxcerol C
Catalog No.:BCX1218
CAS No.:63180-02-9
- Sinocrassoside C1
Catalog No.:BCX1219
CAS No.:909803-26-5
- 7,8,3',4'-Tetrahydroxyflavanone
Catalog No.:BCX1220
CAS No.:489-73-6
- 7'R,8'R-2,2'-Dimethoxy-4-(3-hydroxyl-propenyl)-4'-(1,2,3-trihydroxy-propyl) biphenyl ether
Catalog No.:BCX1221
CAS No.:515813-60-2
- 7,3',5'-Trihydroxyflavanone
Catalog No.:BCX1222
CAS No.:847375-46-6
Design, synthesis, and biological evaluation of low-toxic lappaconitine derivatives as potential analgesics.[Pubmed:36162215]
Eur J Med Chem. 2022 Dec 5;243:114776.
The C(18)-diterpenoid alkaloid lappaconitine (LA) is a non-addictive analgesic used in China. The toxicity (LD(50) = 11.7 mg/kg) limits its application. Two series of LA derivatives, including amides and sulfonamides (1-93), were designed and synthesized by modification on their C4 acetamidobenzoate side chains in this work. In vivo analgesic activity and toxicity of all derivatives were evaluated, and the structure-activity relationship was summarized. Six lead compounds (35, 36, 39, 49, 70, and 89) exhibited approximate analgesic activity to LA but with significantly reduced toxicity. The therapeutic index of these compounds is 14-30 times that of LA. In vivo metabolism study of the lead compounds 39, 49, 70, and 89 were conducted by UPLC-MS(E), indicating the reason for the low toxicity of the potential derivatives might be they are difficult to metabolize to toxic metabolite N-Deacetyllappaconitine compared to LA. The effects of lead compounds on sodium channels and hERG channels were also studied by ion channel reader (ICR) which further revealed their analgesic and toxicity-attenuating mechanisms. Sodium channel assay revealed that the analgesic mechanism of these lead compounds was inhibiting the Na(v) 1.7 channels. Taken together, compound 39 was provided as a new analgesic lead compound with significantly low toxicity and comparable activity to LA.
Analysis of Electrophysiological Mechanisms of N-Deacetyllapaconitine Monochlorhydrate, the Main Metabolite of Lappaconitine Hydrobromide.[Pubmed:35739330]
Bull Exp Biol Med. 2022 Jun;173(2):219-223.
In in vitro experiments on isolated rat hippocampal neurons, we studied the electrophysiological mechanisms of the antiarrhythmic effects of N-Deacetyllappaconitine monochlorhydrate (DALCh), active metabolite of lappaconitine hydrobromide (allapinin). Electrical activity of neurons was recorded by the patch-clamp method in the whole cell configuration. It was shown that DALCh increased the duration of both slow and fast depolarization phases and decreased the amplitude of the action potential. DALCh effectively inhibited transmembrane currents of Na(+) ions and partially K(+) ions through the corresponding transmembrane voltage-gated ion channels. Thus, DALCh, in contrast to lappaconitine hydrobromide, belongs not to 1C, but to the 1A class of antiarrhythmics according to the Vaughan-Williams classification.
Assessment of in vitro cardiotoxicity of extract fractions and diterpene alkaloids from Aconitum leucostomum Worosch: A short communication.[Pubmed:28104561]
J Pharm Biomed Anal. 2017 Apr 15;137:84-89.
Aconitum leucostomum Worosch is a traditional Chinese medicine (TCM) and has a broad spectrum of health effects, but with a narrow therapeutic window. It is important to identify both the therapeutic ingredients and the toxic components to better utilize this TCM. The present study investigated the cardiotoxicity of the selected compounds in Aconitum leucostomum Worosch. The effects of extract of A. leucostomum Worosch and the isolated compounds on cardiocardiomyocytes were evaluated in vitro. Five known compounds in this TCM, including three C(18)-diterpene alkaloids, lappaconitine (2), N-Deacetyllappaconitine (3), and ranaconitine (5), and two C(19)-diterpene alkaloids, delvestidine (1) and anthranoyllycoctonine (4), were isolated from A. leucostomum Worosch. The cardiotoxicity of these components and extract fractions, as measured by lactate dehydrogenase release and apoptosis, was ranked as follows, in descending order: delvestidine>anthranoyllycoctonine>pH 4 fraction>pH 8 fraction>aconitine>N-Deacetyllappaconitine>ranaconitine>lappaconitine. The cytotoxicity of these compounds was shown to be dose-dependent, with delvestidine (1) and anthranoyllycoctonine (4) being the two most toxic compounds to cardiomyocytes in our assays. These results provide a basis for future rational use of this TCM, reducing side effects while retaining therapeutic effects.
A model study toward the total synthesis of N-deacetyllappaconitine.[Pubmed:16238304]
J Org Chem. 2005 Oct 28;70(22):8739-42.
[reaction: see text] A model study leading to the preparation of the AEF rings of N-Deacetyllappaconitine is described. The conjugate addition to the alpha-alkyl cyclohexenone 10 proceeded with high diastereocontrol. The Mannich cyclization of 16 to 4 was accomplished by heating with Rexyn-300 and Na(2)SO(4).
A QSAR analysis of toxicity of Aconitum alkaloids.[Pubmed:15548242]
Fundam Clin Pharmacol. 2004 Dec;18(6):699-704.
Biological activity of Aconitum alkaloids may be related to their toxicity rather than to a specific pharmacological action. A Quantitative structure-activity relationships (QSAR) analysis was performed on the following two groups of alkaloids: compounds with an aroyl/aroyloxy group at R(14) position (yunaconitine, bulleyaconitine, aconitine, beiwutine, nagarine, 3-acetyl aconitine, and penduline), and compounds with the aroyloxy group at R(4) position (N-Deacetyllappaconitine, lappaconitine, ranaconitine, N-deacetylfinaconitine, N-deacetylranaconitine). The LD(50) (micromol/kg) of the 12 alkaloids were obtained from the literature. LD(50) was significantly lower in group 1 than in group 2. The steric and core-core repulsion energies were significantly higher in group 1. The total energy and heat of formation and electronic energies were significantly lower in group 1. The reactivity index of N, C1', C4' and C6' were similar between groups. The reactivity index of C2' was significantly higher and the reactivity index of C3' and C5' were significantly lower in group 1. Log P and pKa were similar between groups. Molecular weight was significantly higher in group 1. A significant linear relationship was observed between log LD(50) and either analgesic log ED(50) or local anesthetic log ED(50). The LD(50)/analgesic ED(50) obtained from average values was 5.9 for group 1 and 5.0 for group 2. However, the LD(50)/local anesthetic ED(50) was 40.4 and 318, respectively. The study supports that the analgesic effects of these alkaloids are secondary to their toxic effects whereas alkaloids from group 2 are susceptible to be further studied as local anesthetic agents.
Preparative separation of lappaconitine, ranaconitine, N-deacetyllappaconitine and N-deacetylranaconitine from crude alkaloids of sample Aconitum sinomontanum Nakai by high-speed counter-current chromatography.[Pubmed:11833641]
J Chromatogr A. 2002 Jan 18;943(2):219-25.
Analytical high-speed counter-current chromatography (HSCCC) was used for the systematic selection and optimization of the two-phase solvent system to separate alkaloids from Aconitum sinomontanum Nakai. The optimum solvent systems CHCl3-MeOH-0.3 M/0.2 M HCl (4:1.5:2, v/v) thus obtained led to the successful separation of lappaconitine, ranaconitine, N-Deacetyllappaconitine and N-deacetylranaconitine from 60 to 500 mg of crude alkaloid sample by preparative HSCCC separation.
Cardiac effects of lappaconitine and N-deacetyllappaconitine, two diterpenoid alkaloids from plants of the Aconitum and Delphinium species.[Pubmed:9491764]
Planta Med. 1998 Feb;64(1):22-6.
Aconitum and Delphinium alkaloids are currently under investigation in search for new analgesic and anti-inflammatory drugs. It has been reported that the analgesic compound lappaconitine (LA), a C19 diterpenoid alkaloid from Aconitum sinomontanum nakai is an inhibitor of tetrodotoxin-sensitive, voltage-dependent sodium channels. In the present study we investigated the cardiac effects of LA and its metabolite N-Deacetyllappaconitine (DLA) in electrically stimulated left and spontaneously beating right atria isolated from guinea-pig hearts. In all experiments, equieffective concentrations were larger with DLA than with LA. At a stimulation frequency of 2.5 Hz the time constant for the onset of LA effects (tau = 56 +/- 29 min) was markedly larger than the one for DLA effects (tau = 14 +/- 8 min). The compounds exerted a significant negative inotropic action at 0.06 microM (LA) and 0.2 microM (DLA). Asystolia of right atria occurred at 4.5 microM (LA) and 10 microM (DLA). Therefore, cardiotoxicity of LA and DLA was much lower compared to aconitine, which caused arrhythmia at 10 nM in our model. For both alkaloids a use-dependent mode of action could be demonstrated. In addition, preincubation with 0.3 microM LA prevented arrhythmia induced by aconitine or ouabain. We conclude that lappaconitine is a naturally occurring compound with class-I antiarrhythmic action.
Effect of diterpenoid alkaloids on cardiac sympathetic efferent and vagal afferent nerve activity.[Pubmed:7498298]
Eur J Pharmacol. 1995 Sep 5;283(1-3):103-6.
The diterpenoid alkaloid, lappaconitine, at a dose of 150 micrograms/kg (i.v.) increased cardiac vagal afferent nerve activity (16.2%) and reduced cardiac sympathetic efferent nerve activity (12.5%). A polar analog, N-Deacetyllappaconitine, at this same dose, increased cardiac vagal afferent nerve activity (40%) and reduced cardiac sympathetic efferent nerve activity (23.5%). Both of these agents also reduced arterial blood pressure and heart rate. A larger dose of lappaconitine (300 micrograms/kg i.v.) produced the same changes in nerve activities and cardiac function as the lower dose. Two other structurally related agents, lycoctonine and aconine, failed to alter these variables in doses up to 300 micrograms/kg. These data suggest that certain diterpenoid alkaloids activate autonomic reflex receptors, including cardiac reflex receptors. The polar agent, N-Deacetyllappaconitine, appears to be more effective on cardiac reflex receptors than the non-polar agent, lappaconitine. Such agents may be useful in the treatment of hypertension.
N-oxides of some norditerpenoid alkaloids.[Pubmed:7673939]
J Nat Prod. 1995 Jun;58(6):929-33.
Eight new N-oxides [1-8] of the norditerpenoid alkaloids aconitine, ajacine, delphinine, delphisine, deltaline, heteratisine, lappaconitine, and N-Deacetyllappaconitine have been prepared with m-chloroperbenzoic acid. The structures of these compounds were established on the basis of their spectroscopic data (1H, 13C, DEPT, COSY, HETCOR, and selective INEPT nmr experiments). The complete nmr chemical shift assignments for all eight N-oxides are reported. Table 2 shows the differences between the 13C-nmr shifts of the N-oxides compared with those of the parent alkaloids.
Lappaconitine and N-deacetyllappaconitine potentiate footshock-induced analgesia in rats.[Pubmed:2008153]
Life Sci. 1991;48(14):1365-70.
The effects of lappaconitine (LA) and N-Deacetyllappaconitine (DLA) on footshock-induced analgesia (FSIA) were studied by the rat tail flick test. Rats subjected to 90 s nonescaping footshock had a significant increase in tail flick latency. Naloxone (4 micrograms, i.c.v.) partially antagonized the FSIA. After 5 consecutive exposures to footshock, rats developed a complete tolerance to the FSIA. The rats tolerant to FSIA showed a cross-tolerance to morphine- but not LA- and DLA-induced analgesia. Administrations of subanalgesic doses of LA and DLA potentiated the FSIA in both intact and adrenalectomized rats.
Roles of periaqueductal gray and nucleus raphe magnus on analgesia induced by lappaconitine, N-deacetyllappaconitine and morphine.[Pubmed:2275382]
Zhongguo Yao Li Xue Bao. 1990 Mar;11(2):107-12.
In the rat tail flick test, ip LA 6 mg/kg, icv DLA 60 micrograms and icv or with morphine 5 micrograms exhibited significant analgesia. But with either LA 40 micrograms or DLA 60 micrograms was inactive. Naloxone (4 micrograms icv) which antagonized morphine analgesia failed to alter the analgesia induced by LA and DLA. Microinjection of DLA 20 micrograms or morphine 5 micrograms into the periaqueductal gray (PAG) or nucleus raphe magnus (NRM) produced markedly analgesic activity. The effects of electrolytic and kainic acid (0.8 micrograms) lesions of the PAG and NRM on the analgesia elicited in the rat from ip LA, icv DLA and morphine were also evaluated. No change in baseline tail flick latency was observed following lesions of the PAG and NRM. But lesions of the PAG and NRM significantly attenuated the analgesia mediated by LA, DLA and morphine. These results suggest that supraspinal sites, especially the PAG and NRM, are involved in the analgesic action induced by LA, DLA and morphine.
[Effects of reserpine and 5-HT on analgesia induced by lappaconitine and N-deacetyllappaconitine].[Pubmed:2403008]
Zhongguo Yao Li Xue Bao. 1990 Jan;11(1):14-8.
In the rat tail-flick test it was shown that ip lappaconitine (LA) 1-6 mg/kg, N-Deacetyllappaconitine (DLA) 4-10 mg/kg or icv DLA 20-60 micrograms/rat exhibited a dose-dependent analgesic activity, but icv LA 20-40 micrograms/rat was inactive. The analgesic potency of ip LA was a little more potent than that of DLA and slightly weaker than that of morphine (P less than 0.05). Combined ip of subanalgesic doses of morphine and LA or DLA produced significant analgesic action. Analgesia mediated by LA was not antagonized by naloxone. The analgesic effect induced by LA or DLA was abolished and restored 3 and 120 h, respectively, after ip reserpine 3 mg/kg. Concomitant administration of 1-tryptophan or 5-HT as well as premedication of alpha-methyldopa prevented reserpine-induced decrease on LA or DLA analgesia. The elevation of brain 5-HT level by icv 5-HT significantly enhanced the analgesia of LA and DLA. LA- or DLA-induced analgesia was attenuated by pretreatment of p-chlorophenylalanine but this attenuation was reversed by icv 5-HT. p-Chloroamphetamine also markedly reduced LA- or DLA-induced analgesia. It is concluded that the central serotoninergic system is involved in the modulation of LA- or DLA-induced analgesia.
Studies on the metabolism of lappaconitine in humans. Identification of the metabolites of lappaconitine in human urine by high performance liquid chromatography.[Pubmed:2310842]
Biomed Chromatogr. 1990 Jan;4(1):43-6.
The metabolites of lappaconitine in the urine of humans having been previously administered intramuscularly with lappaconitine hydrobromide were studied using high performance liquid chromatography with electrochemical and ultraviolet detection. The urine was extracted by means of liquid- and solid-phase extractions. Each of the metabolites of lappaconitine was purified by high performance liquid chromatography on a reversed phase column and identified on the basis of the chromatographic behaviour and the detector response. It was proved that lappaconitine, N-deacetyl-16-O-demethyllappaconitine and N-Deacetyllappaconitine were excreted in urine from humans receiving lappaconitine.


